Updated on August 1, 2019
HairClone Begins Follicle Banking Service
HairClone announced today by press release that it has received approval by the Human Tissue Authority (HTA) in the UK to begin banking patients’ hair follicles. I have also received direct information regarding the status of HairClone’s prototype cell therapy and when injections could become available to patients.

Some statistics about HairClone’s banking procedure have been updated since we last heard. According to the media publications today, HairClone intends to extract about 100 hair follicles from a patient to be put into deep freeze or cryopreservation. These follicles will then be utilized to create cellular injections in the future by dissecting a certain part of the follicle, called the dermal papilla, and then multiplying those cells in culture. The multiplied cells are then re-injected to patient’s scalp in a familiar scenario. The initial procedure of banking one hundred follicles will cost around £2,000 and then an annual fee of £100 to store the follicles.
Direct From HairClone
Beyond the information that was announced in the mainstream today, Paul Kemp has given me some insight into the current activities of HairClone to share with the readers. First off, Paul wanted to mention the important bullet points of the HairClone procedure:
- It will be available to both men and women
- 银行将允许多种注射治疗rom one initial surgery
- Patients with banked follicles will be first in line for cell expansion when treatments are authorised
- The follicles remain the property of the patient so could be used in other treatments if the patient prefers
HairClone tells me that it in order to gain regulatory approval with the HTA for banking the company had to carry out extensive validation studies to show that they could reproducibly extract human follicles, cryopreserve them, ship, test, and thaw them, then extract viable cells that could be culture expanded.
Paul Kemp, CEO of HairClone details“we first submitted the licence application in January and then there was a series of questions some of which needed additional work but the result is an incredibly well understood robust process so the time was well spent.”I asked Paul if this meant that HairClone had to provide cultured DP cells in their test with the HTA to which he responded:
“Yes we did have to show that we could culture the cells to a level that we considered would be used for a treatment. We have done that repeatedly on numerous samples”
I also asked Paul another question which I believe has been lingering on the minds of many readers, does HairClone have a cellular protocol in place? He confirmed, yes HairClone does have a cell culturing protocol, in other words a first generation cell therapy is waiting in the wing now.
When Injections Could Begin
On to the important stuff that everyone wants to know about:the cellular injections. A point of emphasis, Paul tells me that HairClone is not permitted by the HTA to offer injection treatments at this time. In order to offer injections HairClone would have to create a GMP licensed facility which would be approved by the HTA. GMP certification basically means the facility would have to adhere to very high quality of standards.
It takes a considerable amount of money to develop a GMP facility and HairClone was planning on using the money from itscrowdfunding campaignto fund this endeavor, however that campaign did not meet its goal. HairClone is now obliged to go the venture capital route to raise funds, which is sometimes a more drawn out process. Had HairClone been able to meet its crowdfunding goal back in February they would be in position to begin injections by the end of 2019.
First Patient Has Banked Follicles
Paul Kemp also tells me that the first patient has banked their hair follicles with HairClone as of July 30thand it was none other than himself. This is interesting! We’ve got a CEO who is set to be the first patient treated with the multiplied DP cell therapy injections. Paul said he wanted to time the procedure and understand from a patient perspective what was involved.
To recap, HairClone is now allowed to bank patients’ hair follicles, they have a first generation cell therapy in place, and injections can begin once they have developed a GMP system for cell processing and HTA authorization. This is certainly interesting information and puts HairClone among the forefront of companies who could potentially be in the clinic someday.
I’m looking forward to readers’ feedback on the news especially Paul being first patient to bank follicles. Based on previous cell therapy companies are you hopeful for HairClone?
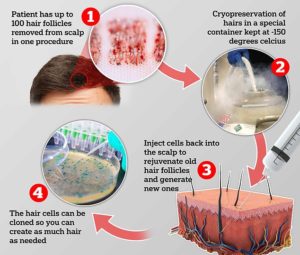

£2000 just to remove 100 follicles, how do they justify that?
My understanding is this involved the extraction, packaging/cold shipment to cryo facility and beginning fee of banking. As far as I know there are similar fees for banking cord blood or other similar tissues.
Paul Kemp does frequently check these articles and would most likely respond to comments though, to clear things up.
I think Dr. Farjo actually quoted a figured of £2500 in a DailyMail article published in January…
Yes, that’s true but the press release from today which Paul emailed me states “around £2,000”
It’s unclear, but perhaps the procedure is between 2000 and 2500 and wanted to be on safe side with initial estimation.
Either way, it’s a sum I’d be billing to pay.
Assuming the treatment worked and/or they successfully developed cloning.
It’s only for people in uk not for others??
Thank you Admin for quick write up still we have to continue the waiting game
Yes, Kapil there is still a wait for injections, however there was an opportunity to speed up the process if everyone wanted to give it a shot. *For the record, there is no blame here just offering an interesting perspective.
只花了3000人(不是捐赠)£投资100 for HairClone to meet its goal and commission a GMP facility. I noticed many people, probably bought Trinov when it first launched for the price of around £100. Now that one bottle is used and gone. HairClone would at least have been able to begin work offering experimental cellular injection therapies for the same price. Of course, there is no guarantees for HC either, but now in hindsight it’s just something to think about.
Yes you are absolutely right we missed the opportunity
@Joe, actually the treatment would have to take place in the UK if/when it’s approved, but patients can be from anywhere in the world. Which is why HairClone has created a network of physicians to supply patient’s follicles. Names like Dr. Jerry Cooley and Dr. Knudsen of Australia involved. see herehttp://hairclone.me/clinical-partners/
很好的进展。我很尊重。Paul Kemp and his team. They’re awesome with the transparency they provide and they have built a network of best scientists and clinical practitioners.
I wanted to but did not contribute to the crowdfunding campaign because I didn’t understand on what basis will people bank the follicles without having proof of the treatment working effectively and safely? Also, a company other than Haircclone using cryopreserved follicles will have its own protocol requirements and may not accept the follicles banked with HairClone. I feel the maintenance treatment instead of follicle bank should have been first step to begin with.
So no Shiseido update? Right..
Thanks John, that’s still coming
Admin please give US your Shiseido Update? When will You Upload it? We Are waiting for so long… Thank You so much!
Is this banking for people who have hair or also for bald people?
Thanks for your update. This is good news and the cost is secondary. The procedure is available now and that’s what’s important.
I would just like to express my gratitude to Paul Kemp and his hard working team.
The hindsight question is interesting. But my view is if 300k is the difference between bringing the most significant hair growth treatment in the world to market or not they would have raised 3m. Look at what Shiseido paid replicel for a treatment that showed negligible improvement in one trial. I wouldn’t be kicking your selves just yet. The Trinov comparison is a little unfair in my opinion. The whole cosmetic industry prays on people’s insecurities to line their pockets. And probably less than 1% of cosmetic products make any tangible difference. So if a product comes out saying it can reverse 5 years of hairloss people are going to jump on it as it is here now. The fact that it seems to be a snake oil is unfortunate for those who have bought it. But I wouldn’t give this credence it hasn’t earned just because it’s cellular based. Sorry to be a miserable git again.
Welsh, I appreciate your contributions. The funny thing is when commenting on a situation like this there is always going to be multiple groups of perspectives on it.
Of course, it’s a given that the treatment is not guaranteed to work yet, (minox, fin, trinov are not guaranteed to work on every individual either), I’m not giving it credence, but the general consensus by the majority here is that £100 is a trivial amount to contribute to find out if an effective cell therapy works or not. For some people the £100 investment is too much to risk, other people would be willing to pay for the whole treatment if they could do so just to try. It’s not a one perspective-fits-all thing.
In the end though, it almost seems like a catch-22 situation will be imminent because, there will continue to be complaints and dissatisfaction that newer better treatments are not available, but also hesitance to support a credible concept until its proven on a large scale. So, in the end we get more waiting. For my personal situation, I wouldn’t be one of the first people to pay for the banking/injections but the £100 investment to get a look at it was worth it.
Correct me if I’m wrong, you would pay the 2000 GBP for follicle extraction then banking but that doesn’t guarantee the treatment will work and/or they they will get necessary funding ? If so, I’d risk a few more years of “older follicles” to see if this actually pans out.
Somehow, read it incorrectly you did. (Yoda talk) I said clearly for my situation (and location) I would support the crowdfund to get it going but not be one of the initial bankers/patients.
Great and interesting news. They didn’t mention the location of source hair did they? Their diagram shows the source follicles coming from the crown. If those were destined to eventually fall out, they would be a bad place to pick from & clone right?
I think its safe to assume that they would only extract follicles from nearer the nape of the neck in the ‘safe zone’.
这是无限的头发吗?我不理解process
So this is more for young people in the long run. Is it any good for someone who wants hair growth in the short term or say as an alternative to a transplant. If you are going to freeze the cells to assist you in the long run, perhaps there may be other treatments in that period for a quick fix or which work out cheaper.
It is such a shame the crowdfunding campaign failed. I personally had pledged money.
Still, we can only look forward.
There are few questions I’d like to ask Paul (if he see’s them and is willing).
1) How long until they expect their GMP approved facility to being up and running?
Do they have a facility already but just need to have it ‘signed off’ and approved?
Or do they still need to acquire/build/equip a facility?
Obviously the former means they are closer to offering treatment.
2) Any rough indication on the price yet?
I know previously Paul was hestitant to answer this or even offer an estimate.
If I remember correctly, Dr. Farjo estimated that each treatment would be around the same price as a hair transplant. Which in the UK can be upto £20,000.
3) I remember Paul mentioning before that a Phd student in Dr. Higgins lab was researching the physiological differences between hair in balding and non balding regions. I believe a lady (Ms Summik?) presented a poster in this years World Hair Conference.
Was there any valuable insight garnered from this research?
A few not so straight forward questions to answer there but any kind of response would be appreciated and helpful to all who I read this.
Thanks
Actually think it’s really cool that kemp is doing the treatment himself. Never heard of this before. If it works for him it’s probably going to work for anyone! None of the treatments in pipeline, especially cloning 1s are guaranteed to work but would be nice if it turns out good for Hairclone. Guess we gotta wait and see and keep fingers crossed.
That’s a great point KD. The acid test will be if it grows hair on Paul Kemp’s head. He is actually the clinical trial.
好的,我们知道:HairClone可以同行高频, they can extract DP cells and multiply it efficiently for treatment and they have regulatory approval for that.
What we don`t know : has anyone tried to reinject cultured cells back to the scalp, do they lose hair generating properties and when will Paul Kemp do it?
Kemp in 2006:https://www.independent.co.uk/news/uk/this-britain/i-have-the-cure-for-baldness-though-it-hasnt-worked-for-me-obviously-6102956.html
http://www.farjo.com/research/hair-cells-regeneration-intercytex/
Intercytex trial results
Nice contribution Benji, thanks. So it appears from this article Paul Kemp used a more primitive version of dp cell injection back in 2006. Is the date on this article correct? I imagine it is, but it talks about a phase 2 trial in 2006. That just seems long ago.
Only real question here is does the process of multiplication cause DP cells to lose their regenerative abilities. It happed to Intercytex and to large extent to RCH 01 (first trial), that is why there is not any updates I’m afraid.
Whole cryopreservation thing is not that important.
That HairCell photo that you showed is first EVIDENCE that impressed me, all the rest are hearsay atm.
Thanks
I agree faust, most important thing here is whether the dp cell injections work. That’s the headline that most of the audience is really after. Like every situation it’s a wait to find out. We can all watch comfortably from our laptops.
p.s. I didn’t forget about HairCell either, work in progress
I have to ask… How much is the cell banking going to cost? Will there be a recurring charge?
The media articles/press release says around £2000 initially and 100 per year afterwards.
That’s not bad…. Once I get a more adult job that is lol
Haha you’re a good guy man. Even better by the time the job comes around you’ll probably be able to review initial HairClone results.
I just think it’s the same procedure that didn’t work before that’s being re-marketed.
的,虽然是什么?没有t even any money to be made in that situation. You really think Bessam Farjo would do that too? There’s no proof that the treatment works yet, but I just don’t see that being the case.
“…their subsequent Phase II testing on humans failed to produce cosmetically significant hair regrowth. It’s suggested that the key to success may lie in growing stem cells in vitro to a hair follicle stage and then transplanting the hair follicles using today’s state of the art surgical hair restoration techniques”.
” Their stock which at one time traded for over $100 per share has slumped down to $4 per share and is now virtually worthless since the company is being dissolved.”
https://www.regrowhair.com/intercytex-takes-a-dive-the-end-of-hair-multiplication-cloning-research/
They get £2,000 a pop from people that fall for it.
Originally they were trying to grow new hair using DP cell injections.
This didn’t work but what they discovered was that the DP injections revived/rejuvinated existing follicles thats had miniturised.
This rejuvenating property of the DP cells is the basis of the treatment being proposed.
So technically they failed to achieve the original objective but they discovered they could rejuvenate existing hair.
From the ashes of intercytex, Anderans Research Institute was born. Then they too ran out of money.
Now Hairclone have picked up the baton are attemping to continue the run using the fundamental concept of hair rejuvenation using DP cells.
I’ve heard this too Matt. New approach is for rejuvenating existing follicles, slightly diff than what intercytex/aderans were after. Also I reckon there has been increased in understanding in dp cells since 2009??
When I see Paul Kemp with a full head of hair, then I’ll buy into what he’s selling.
Just for hahas, is there any company/treatment that appeals to you at this point?
Only the ones being looked at by people with a solid scientific background, proven results in their field, and an aversion to hype. Terskikh and Tsuji are the only people that seem to fill those criteria.
2025 can’t come soon enough
Can you answer my question
According to what I’ve read, the initial treatment aims at thickening existing hair, then later maybe they can develop a treatment that grows new hairs. But first things first.
realistically every company we heard of is in the same boat working on this. some make it out like one company has a better shot than others but there’s no proof to that. thats just ‘hype’ too. proof comes when photos and stats are out.
Laboratory study paves way for new approach to treating hair loss in humans
Prof. Fukuda, Yokohama University
https://medicalxpress.com/news/2019-07-laboratory-paves-approach-hair-loss.html
Years and years away Ahmed
Most interested in when the injections are first going to be tested on humans. also what is the science that progressed on the cells since the first attempt from years back?
I’d like to hear Yoda’s top 3 potential treatments in the pipeline then, with the timeliness of market release being a factor…
Alreday Fewday week end Shieido will update today??
Jwwww, thanks for keeping up on things. The Shiseido update was planned for this week but the Hairclone news popped up unexpectedly so wanted to cover that first. Didn’t intend for it. Just to ease things up a bit please understand Shiseido still has a ways to go before any product release.
Thz for reply,That main the news delay to other few day?
Yes, this week for sure.
Hi Admin, what happened to Medipost’s Jay Lee he normally answer your questions
Hi Kapil, I believe he is still keeping track of comments on the board, he may answer your question if you post it in the older articles. The latest clinical trial is ending Sept. 18th.
Admin, when you say “still has a ways to go” how long do you mean? The lifecycle for rch-01 is practically over so apart from building a brand for the product and logistics what could take more time?
I do not know how long the product has to go I just know that it is not going to be released in the near future. The Shiseido update is scheduled to post later tonight.
Really? Not even later this year or next year? That sucks. Thought it said 2019/2020 for Shiseido on your cures updates page but I guess that means something else?
RCH 01 is becoming a bit like the Monty Python dead parrot sketch.
Relatively speaking, is it easier to accomplish what hairclone is doing compared to what stemson is doing etc? Does anyone know?
Thanks I have gone through old article yes they are doing a bigger trial with twice a day application which was planned for 24 weeks in June last year it’s over a year no problem we can wait one more month for even better result
管理员,谢谢你向我澄清ome misinterpretation. It shows you care and is appreciated. Internet hair loss blogs and forums can be a brutal place (as you know all too well). It’s a big reason why I rarely post and am hesitant to share treatments. This despite me fighting this disease for longer than many readers may be old (I started losing hair at 17, I’ll be 57 this year). While my hair is not “perfect’ it’s decent and I notice the thinning more than anybody else. Ladies and gentlemen, we are in this fight together. We can disagree with the Admin or each other, however we can do this in a civil, respectful way. We are fortunate to have sources of info like this site, resources I didn’t have 40 years ago when I started to lose hair. Thank you Admin, keep the faith, remain hopeful but be realistic.
Thanks Yoda I appreciate the kind words from a hair internet vet like yourself, it means a lot. Was hoping to get your views on top 3 upcoming treatments as well. We will have time tho, whether in this article or next lol
Last June Lee Buckler tweet Shiseido announcement in Sep/Oct almost year passed…..
Yoda, I have a similar story. (Started at 14) but I’m only eighteen now. Were you able to preserve the majority of your hair in the years after you started losing it? And if so, what drugs did you use for it? My regimen is currently 0.1fin and 7%minox (topical). Wondering what you used for your hair and if you think the topical finasteride is going to help at all. Thx
Greg, I feel it buddy, I was loosing hair at age 18 as well. It’s tough at any age but younger hair loss can be painful. It’s hard for me to advise anyone, I’m not a medical professional plus everyone is different. I started out over 30 years ago with 2% minox and it worked great for a while. Long story short, over the years I had to increase the concentration of minox to 15%, this also has topical fin with some other goodies. I also started oral fin as well around 25 years ago. I’ve moved on to Dut about 4 years ago, fin started to loose it’s effectiveness. I added RU about 2 years ago and it gave me an extra boost, however I would never recommend unregulated Chinese chemical’s to anyone. While I have experienced no appreciable side effects from this “kitchen sink” approach, I would urge you to move slowly. Be on your current treatment for at least a year before deciding to up the minox concentration. I can’t speak to an 18 year old taking oral fin, I’d ask your doc about that. Hope this helps, keep the faith, investigate all the options after you give each treatment some time.
Thanks for the advice, Yoda. I guess I’ll keep on my current regimen for now but with new treatments releasing relatively soon I think many of us will drop our regimens. In your opinion, what’s the most hopeful upcoming treatment and when do you think it can hit the market? Even if it’s only in one country? Thx again
哇管理员,您希望尤达的意见? ?不要让screen name fool you, I’m not all that wise.
I have no horse in the race as far as “top” treatments. The ones that primarily get my attention are the treatments that seem to have solid financial backing, strong research teams, close to market, will be accessible and probably priced at a sensible level. This includes Follica, Saumed and Cassiopiea (forgive me if any or all are spelled incorrectly). Not saying that these 3 are definitely coming to market and one’s I didn’t mention aren’t, but to me these three seem like they have the closest, near term potential to be out in the next couple years.
Of course I wanted the opinion of a person named Yoda lol. Your top picks are interesting and make sense. Follica could actually be out next year which puts it furthest along in North America. If only one of these cosmetic topicals could turn out to be effective, it would really bridge the gap for everyone.
Too few too expensive too late. Would have been great if it was ready back in 2009. It would make sense. With a bunch of treatments reaching market within 5 years (Samumed, Breezula, Histogen, Follica, …) why would we store follicles in a fridge ?
The fact that Dr Farjo is on-board, gives HairClone a lot of credibility for me. Still, like a lot of the other comments here, I would be happier knowing that the treatment has an end product, before spending the money on banking the follicles.
嗨,谢谢你所有的问题和评论s, even the sceptical, negative ones. We realise that we can’t please everyone and these comments are helpful for us to clarify our message. Our values at HairClone are openness, honesty and fairness and all the Founders have given thousands of hours of our time for free because we really believe in the potential of the treatment and in what we are trying to build. My answers are long as it is critical that we get our message across and your feedback is really very helpful in doing that.
There have been some questions about the cost of the banking. This has not yet been completely fixed, but the HairClone figures discussed here and in the press are in the right ballpark. This will cover the cold chain shipping, testing, cryopreservation, documentation ( a LOT of documentation) and one year’s storage, all to a quality level that will allow the follicles to be used clinically later. There will then be an additional annual charge for continued storage and these charges does not include the surgical fees to extract the follicles as different clinics in different regions will have different cost structures.
As Follicle Thought says, this is similar to fees paid for banking of cord blood and tissue but there are FOUR huge differences between those types of tissue banks and what we are doing at HairClone.
1) In our case, ALL of the profits from banking will go to development, authorisation and licensing of the treatment system. The more patients banking, the faster we can get to the treatment stage. Other tissue banks just store tissue and hope that some other Company develops treatments that will need the stored tissue. That being said these follicles will remain the property of the patient and could be used by other Companies.
2) Patients banking follicles with us have already undergone the follicle extraction stage and their follicles with have been tested and ready for the cell isolation and culturing step by us in the UK when we are ready.
3) We appreciate that these patients banking follicles now have taken a risk in banking before the treatment is available and that in doing this they have supported development of the treatment.
We will recognise this in two important ways. Firstly, they will be first-in-line for treatment when it is available and when they need it. This will be an autologous process and we will be limited initially in how many patients’ follicles we can process. Secondly, we will also give a significant discount for the cell multiplication step to patients banking follicles before treatments are available. At this stage, we don’t yet know what that cost of cell extraction/multiplication step will be and what discount we will be able to offer, but it will be significant and it will make the full treatment cost lower than that being paid by people who waited to bank until the treatment was available.
On top of this, as we have said, banking early “stops the clock” on future ageing of the cells
Someone has said that this banking is like paying a downpayment and getting on the waiting list for a car and that is a pretty good analogy though we still have to show the car works. I would be interested in getting people’s feedback on this as as far as we can see, this is unique in Biotech and we want to make sure people understand it.
Some people, like some of those contributing to this conversation, will want to wait to bank until the treatment is proven and available and that is obviously their choice. We may be wrong, like we were with crowdfunding, in trying to build a “community” and the majority of people may just want treatment, but so far, a great many years have passed and treatments haven’t become available as company after company runs out of funds. We created HairClone to hopefully change that paradigm. Just as importantly, those people that wait to bank until a treatment is available will not have benefitted from the three points noted above especially going 3) and by then the waiting list could be very long.
Some people seem to think that the more equity investment a Biotech raises the better their concept and ideas must be. However, the classic VC investment route has very significant issues associated with it. Large amounts of equity investments can be raised and are, though the competition for this money is huge and fickle and currently cellular immunotherapy for cancer treatment is attracting the vast majority of the VC funds in life sciences at the moment. The team at HairClone have a large amount of experience in many different Biotechs and we know from bitter experience that these investments come with problems, mainly because the VCs want a quick answer and a quick (and large) return on their investment. One of their sayings is “fail fast, fail cheap” and this often ends up forcing the company to rush to clinic and it gives them one shot at a clinical result that would enable them to go to the next stage. That happened with Intercytex and then with ARI and we want to avoid that here. That being said, our unfortunate Crowd Funding effort means that we are now talking to a variety of professional investors but this will take time to complete.
Mattt (Aug 2nd) is correct when he says that this original work of Intercytex and Aderans Research was based on the Jahoda work which showed DP cells injected in rodent skin induced brand new hair follicles but this didn’t translate to the human as Intercytex and ARI found. However, as is often the case with clinical trials, unexpected findings lead into different directions and it appeared that follicles that were newly miniaturising because of loss of DP cells were being rebuilt and the hair shaft dimensions returning to original size. This would agree with the 2003 publication from McElwee et al. and we feel rebuilding is a more accurate term than rejuvenating as our intention is not just a temporary stimulation of hair shaft formation but an actual recellularising and rebuilding of the follicle. Shiseido are looking at just this route but using dermal sheath (DS) cells rather than DP cells. To answer other questions on this thread, this has not yet been shown either by ourselves with DP cells and the Shiseido results with DS cells are not yet public. There are however many patients eager to try and if the crowdfunding had been successful we would have been able to offer clinicians a “cell expansion service” around now and because of the deep surgical insight from our clinical partners we are confident that they would learn how best to use these cells to rebuild follicles. In answer to Matt’s question we are working with a GMP facility that is licensed to produce cell therapies. They would need to do all the documentation and testing to move our lab system to GMP (Did I mention that GMP requires a LOT of documentation?) and we think with funding it would take 6 months to be ready to offer the service.
Creating brand new hair follicles is a much more challenging prospect though several groups, including us, are looking at it. Interestingly, all these groups are coming up with variations on a theme of mass producing follicle rudiments or hair germs or protohairs created by making 3D beads of a combination of cells such as DP cells that control the epithelial cells that in turn make the hair follicle and later the hair shaft.
We see three types of patients that could benefit from these treatments
Patients with miniaturising follicles that can be rebuilt by DP cell injections. By treating these patients every few years as the “wave” of hair loss progresses, their appearance would be maintained
Patients with a combination of hairs that can be rebuilt and others that have miniaturised too far to be rebuilt. Here they could be treated by a combination of DP cell injections in areas that can be rebuilt and traditional hair transplant in areas that cant be rebuilt. Together these would cover more area than transplant alone
Those patients with insufficient hair to transplant and hairs that have miniaturised too far to be rebuilt. These would benefit from the brand new hair follicles but it must be stressed that this is a much longer and more challenging task than 1) or 2) and the treatment would be much more expensive to produce
I must stress at this point that I was just the first banking patient and haven’t been treated with cells as we aren’t authorised to do that yet. Plus I need again to stress, our first treatment will be to rebuild miniaturising hairs not generate new hairs and my hairs have been miniaturising for around 40 years and have probably gone beyond the point that they can be rebuilt.
I was the first banking patient as I wanted to test the system, time the clinical part and make sure we had all the paperwork that we needed to be able to train the new clinics. In doing this I was also an example of why banking earlier is better as at 63 years old my follicles were very fragile to handle.
In conclusion I just want to say that the banking is a means to an end and not a treatment in itself. Most importantly, we are not doing it as a “get rich quick” scheme but a very genuine first step towards development of a treatment.
Hi Paul,
Thankyou for responding to my question,
And also for taking the time to provide such a detailed response. No doubt most of us really appreciate your transparency and honesty.
I have many more questions I’d like to ask but I’ll stick to just two if you would be willing to answer them.
1) You say the term follicular rebuilding is more accurate and that you’re aiming for recellurisaring.
In simple terms, would this mean that ‘rebuilt’ follicles could become ‘dht resistant’ and somewhat immune to further miniaturization?
2) At what stage is a follicle too far miniaturized to benifit from this and be ‘rebuilt’?
Is there any previous data from the intercytex/ARI that can help answer this?
Thank you
in answer to your question 1). Yes we hope so. No data yet but wouldn’t that be amazing!
In answer to 2) we dont know yet and the only quantitative data comes from Bruce Morgan’s lab who showed rodent follicles could recover if less than 1/2 of the DP cells were removed
Hi Paul,
Thanks for responding, one last question from me;
What is the relationship (if there is one) between DP cell population decrease and the decrease in hair diameter?
Basically, is there a way of comparing a terminal hairs diameter and miniaturized hairs diameter and deducing the % of DP cell loss?
Thanks
Dr Kemp, I really appreciate your time responding here but your answer fails to impress me. The fundamental problem is that your answer is not addressing the question IF you have a treatment which works and IF yes to what extent and finally when would be available (or at least to provide any data or evidence of it). All the amazing discounts we might receive and reduced waiting time if we freeze our cells now sounds like old sales tactic to me, I am sorry if I may sound blunt but I do not see any value in freezing any cells (there would be still a lot of available hairs from the back even if we turn nw7) and I am really struggling to understand why your company is pushing to position this freezing service when you do not have any working treatment(or at least you did not provide any evidence of it). A good logics would be to have priority number one to create a treatment which deliver result and then only after then offering a freezing service in order to avoid repeating the extraction of grafts for cloning purpose. Needless to say, I am really skeptic about the whole thing but hope you prove me wrong.
I wish we were in a position to show the definitive clinical data that you need but we aren’t yet in that position. What we do have is a large amount of published preclinical and clinical data that would support our clinical hypothesis. In order to gain that data we didn’t want to create another exclusively venture funded enterprise for all the reasons I gave above. Banking is a way to involve patients as well as providing the advantages I described above. Yes even NW7 will have enough hair for our treatments but as I have found with my experiences all hair ages.
We are now working really hard to raise the funds needed to develop the data we need but we have lost a year compared to where we would have been had we been successful with crowdfunding
I do respect that Paul as a CEO of a company, comes onto sites like this and answers questions, gives updates.. credit where credit is due, etc..
Surely with the potential returns any investor would jump at this opportunity. Why go through a crowdfunding campaign? Seems dubious.
We tried crowdfunding so that we could involve non-professional investors who had vested interest in seeing a hair loss treatment. There are many many ideas out there with massive potential returns and with shorter timelines and less regulation than medical therapies. As a Venture Capitalist said “Its a buyers market”. There is an interest to fund therapies like ours but it takes a lot of our efforts to find the right investor who wants to invest for the right reasons
Thanks Paul for answering our questions
One thing that seems striking to me is that the treatment is not definitive and that it must be repeated every so often.
How often do you think it should be repeated?
And in the particular case of those who are using Finasteride or Dutasteride,
Do you think that touch-ups would also be needed from time to time or due to the antiandrogenic effect of the drugs would there be a synergistic effect?
We greatly appreciate that you dedicate part of your time to answer our questions.
Good luck!
Our aim is to rebuild miniaturising follicles. Obviously we can’t begin to rebuild a follicle until it has begun to miniaturise hence the need to repeat treatment probably every few years depending on how quickly hair loss is progressing in any particular patient. If we are right (and we dont have the data yet) then each follicle would only have to be treated once
Love how transparent you are being with us Paul I would definately consider if my hairloss wasn’t so aggressive but I dont think a little rejuvenation is going to cut it for me personally. I know you mention trying to create new follicles down the road but that’ll be quite awhile unfortunately. I wish you guys the best.
Hi Paul,
You say that if you’re right, each particular follicle will only need to be treated once.
I presume this means that (if you’re right) any individual follicle that had been ‘rebuilt’ would be immune to future degradation of it’s DP cell population?
So that infers to me that it would be immune to re-miniaturization.
Am I misunderstanding something here? Is there a subtle difference.
I understand you still need data to validate this.
Thanks again,
Matt
I’ve just realised that you’ve essentially answered this with your reply to my questions above,
Apologies,
What is HairClone contact information?
phone number or email
Thank you
feel free to contact me atpaul@HairClone.me
Truth is this will remain one of the most interesting companies in the field until they can inject first few patients to show results one way or the other. There’s not much else beside them. Shisiedo is slow other companies may be too overprice or never come to market.
Is there not a chance to try crowd funding now that we have more information, more people interested, more publicity?
That is a good question for Paul Kemp..
Hi
谢谢你的问题。cr的营销owdfunding cost us a awful lot of time and money and we are don’t really have any indication that there is sufficient interest out there to repeat it. The negativity (including in threads like this) just reinforces this opinion. We did assume that we would have way more interest first time around, as we have over 1,000 people who have signed up for our mailing list and less than 10% of those actually pledged.
Unfortunately, the long time waiting for an effective treatment and the history of Companies like Intercytex, ARI etc has created a chicken and egg scenario of money and data with people hoping someone else comes up with the money to provide the data before they are willing to provide money. We hoped we could break that cycle with having crowdfunding where a lot of people, each providing a little funding at a low company valuation, could be used to allow us to create the data that would in turn increase the company valuation and attract larger chunks of funding etc and early small investors make the profit as well as having early access to a treatment!
Paul, try not to let the negativity bring you down. Some lads comments aren’t worth listening much.
I think more people realize now that the simple crowdfunding bit was a smart and cheap approach and (some) know now it could very well be a missed opportunity. Keep on going and I wish you find success for raising capital with Hairclone.
Hi James
Thanks for the positive comments and yes we are continuing working on “plan B” but as I have mentioned, we are unfortunately now a year (and counting) behind our original timelines. Fingers crossed it won’t be much more of a delay
“We hoped we could break that cycle with having crowdfunding where a lot of people, each providing a little funding at a low company valuation, could be used to allow us to create the data that would in turn increase the company valuation and attract larger chunks of funding etc and early small investors make the profit as well as having early access to a treatment!”
I think that was a great quote actually
Thanks, smile. It was more than a great quote, it was we thought a great plan! With “citizen funded science” doing something that traditional VC funded companies hadn’t managed to downhill hopefully at the same time making these early investors a great return.
I really don’t understand. You have to pay $ 2.000 to freeze 100 follicles to use in a treatment that we don’t know if it works!!
That’s where things are at right now. They are confident they have a therapy which will work, but it needs to be tested in humans to show the proof.
In order for it to be tested in humans they need a could hundred thousand to set up a high standard facility and process. The crowdfund would have taken care of this but not enough people contributed to try it. Now, Hairclone is seeking to get venture capital funding to move to the next step.
As soon as the money is place Hairclone will be ready to test the injections in humans.
Question:
Is this a permanent fix to hairloss (should it work) or treatment you need to repeatedly have?
Other then that, what about the risks of these injections causing any life-threatening problems down the line?
Paul or FollicleThought, any chance either of you could chime in?