Updated on October 25, 2022
Updates – Cures for Hair Loss 2022
A very good idea is to bookmark this article page in your browser or mobile browser for easy access on the latest hair loss cure and hair growth treatment news. This will allow you to check it easily for updates. If you are looking for a corresponding article from a past update, simply use the “Search” function on this site to locate the article. As always, thanks for reading and please share this information with all you feel would benefit from it!
News Feed
Amplifica Completes $11M Funding Round (10/25/22)
On October 24, 2022, Amplifica announced the closing of a $11.8M Series A financing round. The press release also mentions for the first time the development names of Amplifica’s current pipeline molecules; AMP-303 and AMP-506. Inferring fromthe release, these molecules are described as “signaling molecules” which help to kickstart hair regrowth. No firm timeline was given for the initiation of any clinical trial, other than to state that the company expects to begin a trial in 2023. This news represents a wave of activity which has been sweeping through various contender companies in the hair growth space.
Eirion Eyeing New Hair Drug Trial (10/22/22)
Eirion Therapeutics, a company with hair growth and gray hair reversal therapies in its pipeline, issued a press release earlier this week. The release was the first meaningful update from the company in over a year. Whilethe releasewas focused on Eirion’s candidate for facial aesthetics, it also noted that Eirion is planning to file an IND for its topical hair growth candidate ET-02, and hopes to make it to a clinical trial for ET-02 by mid 2023. Another case of positive activity showing up.
Signs Of Activity From BiologicsMD (10/18/22)
After several years of outward silence from BiologicsMD, the company issued a press release today announcing the appointment of Dr. Brett King as Senior Scientific Advisor to the company. Dr. King is well known for his clinical research in alopecia areata and JAK inhibitors, as well as research in general hair loss disorders. BiologicsMD is developing recombinant fusion protein-basedtherapeuticsfor androgenetic alopecia, alopecia areata, and bone disorders. Hopefullytoday’s newsis followed up with further clinical research activity from BiologicsMD in the near future.
Verteporfin Breaking Through? (10/16/22)
The latest update from the verteporfin donor regeneration study at 119 days has been shared by Dr. Barghouthi. Head to the official Follicle Thoughtpage recapping the entire study.Below are some of the latest photo examples shared from the 119 day update, this time from a wider perspective we have not seen before. The visual difference between control areas (left) and test areas (right) is noticeable, although, perhaps slightly zoomed out in the 0.4mg test photo. Share your feedback on the official page linked above.
MolGenBio FK506 Analog (10/6/22)
Follicle Thought debuted a new company this week,MolGenBio的韩国。MolGenBio Yeo J博士成立了oon Yoon, a professor of pharmacy at Seoul National University. The company focuses on editing and enhancing microbial molecules due to their high rate of success in pharmacology. Head to the Articles page to learn more about MolGenBio and their candidate compounds for hair growth and hair loss prevention.
Epibiotech Pig Results & Stemon Inc. Intro (9/27/22)
The latest Follicle Thought article published last week shared some interesting tidbits on EPI-001, the cell therapy in development by Epibiotech. The company participated in a hair loss research conference in Korea over the summer, and also shared details from a preclinical study involving pigs.
Stemon Inc.is a Korean biotech company which has developed an innovative system of cellular reprogramming using ultrasound and culture mediums. They have a research line for creating exosomes tailored for hair regrowth. Read more about both of these companies on the Articles page.
Jupiter Wellness Licenses Minoxidil Booster (9/17/22)
In June 2022, the publicly traded companyJupiter Wellness获得应用生物学、皮肤学集中"D biotech headed by Dr. Andy Goren. Applied Biology was known for publishing extensive research on sulfotransferase enzymes and minoxidl responsiveness. The company also patented a novel SULT1A1 minoxidil boosting product which was previously covered on Follicle Thought called “MinoxiBoost.”
On September 13, 2022, Jupiter Wellness issued apress releasereiterating the licensing agreements in place for its minoxidil boosting topical which include deals with Taisho Pharmaceutical of Japan and Cosmofix Technology and Sanpellegrino Cosmetics of India.
Verteporfin Donor Hair Regeneration Study Update (9/11/22)
Dr. Barghouthi has shared more photos from his FUE extraction study with verteporfin. The subject is now about 84 days out from the original date of surgery. Below is a fascinating piece of data showing multiple hairs which appear to be emerging from an FUE extraction site which was treated with 0.24mg of verteporfin following extraction. For the full update, head to theDonor Healing Study With Verteporfin.
Latest Hair Therapies Article InWIRED(9/6/22)
The mainstream media has once again taken a delve into the hair-loss cure landscape. The latest effort appears in WIRED and is titled “This Follicle-Hacking Drug Could One Day Treat Baldness.” The “hacking” drug which the title refers to is none other than SCUBE3, which we have certainly had our fill of over the past two months. Nonetheless, the article was well researched in my opinion and provides some useful insight into the future timeline of the one of the lesser known companies working on hair growth at the moment: Turn Biotechnologies.
According to thenew articlein WIRED, Turn Bio is eyeing the end of 2023/early 2024 to enter a clinical trial for TRN-001, which is slated to be a protein cocktail injection designed to reset follicular stem cells. It’s an appealing-sounding approach to be sure.
KX-826 Pyrilutamide Phase 2 Results Published (8/29/22)
Kintor Pharmaceutical shared a press release today announcing the long awaited statistics results from their phase 2 trial for male AGA in China. Follicle Thought was also able to discover an elusive poster shared by the Chinese Society of Dermatology which contains further insights into the drug’s trial performance. Head to the main page now to read furtherinformationabout this exciting development.
Bad Hair Day Show Airs On TLC (8/24/22)
A new reality tv series involving dermatologists, hair transplant surgeons, and their patients is airing its first episode tonight on the TLC network. Head to the Articles page to watch a trailer for the series and check your local listings to watch Bad Hair Day. It’s scheduled at 10/9c pm August 24th.
Cutia Therapeutics Advances New Drug To Trial (8/19/22)
Earlier this week, Follicle Thought had the scoop announcing a new drug entering a phase 1 clinical trial in Q3 2022. CU-40101 by Cutia Therapeutics is set to be trialed in male subjects by the year’s end and the study is listed on the Clinical Trials.gov portal. Is this your first time hearing about the article? Make sure you are subscribed for email notifications when new articles drop by entering your email on the right sidebar or bottom sidebar on mobile browsing. You can also bookmark the front page of Follicle Thought for quick access.
Verteporfin Study Showing Signs Of Life (8/14/22)
This morning, Dr. Taleb Barghouthi updated us with new comparison photos of the donor area of his first patient in the verteporfin FUE experimental study. Without going overboard, we can confidently say there are new interesting developments happening in the test FUE extraction sites, namely, the appearance of singular hair follicles sprouting. Below is a photo of the 0.4mg control and test areas. While I won’t claim the single hair in the test area is definitely sprouting from the exact extraction site, and is not a native hair, I can’t help but notice how small it is compared to the donor hairs around it. Is this a regenerated hair or was it there all along? For more photos and discussion please visit theDonor Healing Study With Verteporfinpage.
Philanthropy For Androgenetic Alopecia Cures (8/5/22)
It has dawned on me once again that there should be more done to spread the message of AGA cure research to philanthropists and those with resources. This message needs to be spread by members of the audience as well and not just me. Can you share this message, either directly or paraphrased in your own words, with someone who can make a difference? It is important for more people to get involved now.
There have always been people who have chosen to become benefactors for medical and environmental issues. Despite affecting millions of people, hair loss is still yet to receive a significant contribution from such people. Who could become this great benefactor? Please tag them.
— Follicle Thought.com (Hair Growth Research) (@FollicleThought)August 2, 2022
Approved Drug Repurposed For Hair And Wounds? (8/1/22)
An orthopedic surgeon from the University of Arizona is seeking to repurpose an FDA approved drug, 4-aminopyridine, for tissue regeneration and even hair growth. The drug, 4-AP, was recently featured in a peer-reviewed article demonstrating its effects on wound healing and regeneration in an animal model. A startup company has been established to hopefully test these discoveries in the clinic and benefit patients. The surgeon who is behind this work, Dr. John Elfar, is currently looking for collaborators to further develop the hair growth implications of 4-AP. Head to the Articles page for the full story.
SCUBE3 Research Video (7/19/22)
A video interview featuring Maksim Plikus and his fascinating SCUBE3 research has surfaced from a news organization based in Ohio, of all places. Spoiler alert: Maksim appears to have told the journalist that SCUBE3 is intended to become a therapy which could potentially be “microinjected into scalps to reverse baldness.” Here’sthe videoshared by Spectrum News 1. After clicking “play” click “ask later” to see the video.
Update: Anothervideo interviewof Maksim Plikus has surfaced online.
Best Hair Transplant Resources And Research (7/12/22)
A new article has been shared on the front page detailing the hair transplant industry. The article shares information on hair transplant techniques and gives examples of average and ideal hair transplant cases. Importantly, guidance is given on how and where to find the most reputable surgeons and clinics. Finally, some cutting edge hair transplant research which is taking place in the Middle East is presented in the article. If you’re new to hair restoration, make sure you take a look at this article.
Maksim Plikus Identifies New Growth Signaling Molecule (7/1/22)
Our favorite hair researcher from Southern California has made a notable new discovery. Yesterday, on June 30, 2022, the studyHedgehog signaling reprograms hair follicle niche fibroblasts to a hyper-activated statewaspublishedin the Developmental Cell journal. The study demonstrates that Plikus et al identified the SCUBE3 protein as a potent stimulator of hair follicle stem cells and the hair growth cycle. Plikusdescribedthat SCUBE3 is secreted by dermal papilla cells as a signaling molecule to interact with the neighboring stem cells.
What separates this research from other academia-based findings is Plikus has founded his own biotech company Amplifica, which has already begun raising capital to further develop therapeutic compounds such as SCUBE3.
Exosome Companies Working On Hair (6/15/22)
A new article has been shared featuring two new companies developing exosome-based therapies for hair growth. Exosome science has quietly continued to evolve over the past few years after it made an initial splash in regenerative and aesthetic medicine clinics. Head to the main Articles page to learn more about Rion LLC and Xollent Biotech, two noteworthy exosome biotech companies.
First JAK Inhibitor Approved For Areata By FDA (6/14/22)
You may have seen the news that Eli Lilly’s drug Olumiant (baricitinib) received the FDA’s first approval as a systemic treatment for severealopecia areata.Barcitinib has been an approved drug for rheumatoid arthritis since 2018 and has been prescribed off-label in some cases to treat AA, however, now it receives an official designation. This can be seen as an important milestone for people who live with AA. For those who are interested in other therapies in development for alopecia areata, check out theAA处理管道Articlewhich includes therapies outside of JAK inhibitors.
Kintor Phase 2 Results Delayed (6/1/22)
According to Kintor’s “Roadshow Presentation” on theirofficial website, the highly anticipated pyrilutamide/KX-826 phase 2 results will be delayed until September 2022. Results were planned to be shared at the Annual Meeting of the Chinese Society of Dermatology, but that meeting has been postponed until September 2022. The reasoning behind the delay is due to a recent increase in cases of a certain flu strain in China. September will mark a full year since the original announcement that the phase 2 trial in China was a success. At this point, we could only describe the situation as out-of-the-ordinary. It may be practical to now keep expectations of the KX-826 program very grounded until we finally get the data.
Concert Pharmaceuticals Impresses In Phase 3 (6/2/22)
有几个人花时间给我的顾虑t Pharmaceuticals phase 3 trial press coverage which includes some very noteworthy photography. The only catch is that the drug CTP-543, which is a JAK inhibitor, is intended for alopecia areata and not androgenic alopecia. Nonetheless, this may be a supportivenew developmentfor those seeking treatments for AA. CTP-543 is a modified form of ruxolitinib.
HUTERA Details And OliX Trial Horizon (5/28/22)
Follicle Thought recently conducted a brief intervier with the leadership of Moogene Medi regarding their upcoming treatment HUTERA. Just as the article was being published, another Korean biotech with a program for hair growth, OliX Pharmaceuticals, anounnced they had raised ~$45M USD and shared an update on their timeline to go into a human trial for AGA. Check the front page of FollicleThought.com to read this new article.
A Second Chance For Follicum? (5/17/22)
It’s been just over a year since Follicum announced disappointing results in its phase 2a trial for its topical peptide FOL-005 in AGA. The press release shared onMay 6, 2021stated that the highest dosing group showed a significant increase in hair growth over its baseline levels, but it was not statistically significant compared to placebo. Now, a year later, Follicum’s IP has been acquired through a merger with Swedish biotech company Coegin Pharma. According to arecent press release, CMO of Coegin Pharma, John Zibert, has carried out an in depth analysis with third party experts and determined that the data shows usefulness for FOL-005 in certain treatment groups. Coegin Pharma is now seeking a partner to complete the development of FOL-005 for hair growth. What to make of this? It’s not easy to decipher at this time, but it’s something we will keep an eye on at Coegin.
Monasterium Research In A Nutshell Course (5/14/22)
Dr. Ralf Paus and the team from the Monasterium Laboratory in Germany are hosting a world class training course in hair follicle research this June. The training course will feature a multitude of researchers who will give lectures on specifics aspects of hair biology and therapeutic targets. Visit theMonasterium Educationpage for more information on this course.
Moogene Medi Prepares Ultrasound-Delivered Dutasteride HUTERA (5/7/22)
Remember what I was saying about hair growth innovation and a particular geographical location? On May 2, 2022, Moogne Medi of Korea shared a detailed blog post about their new hair growth therapy in development called HUTERA. The treatment is a formulation of dutasteride which is encapsulated in nanoparticles which are attached to micro-bubble delivery systems. During application, the microbubbles containing dutasteride are forced into the epidermal and dermal layers of the skin via an ultrasonic device. According to Moogene’sblog post, this highly specialized delivery system allows for much higher concentration of dutasteride to be delivered into hair follicles, while also creating much lower concentrations in systemic blood levels compared to oral dutasteride. A further update will be expanded upon.
D.Nature Of Korea Develops JAK/Wnt Cosmetic (5/3/22)
When it comes to Korea and hair growth innovation, you can’t stop them, you can only hope to contain them. And in reality, you wouldn’t even want to do that. On a serious note, a new functional cosmetic product for hair loss prevention/hair growth has beendesignatedby the Korean Ministry of Food and Drug Safety. The product “CMX” aka “Anacell” was developed by the D.Nature company. The active ingredient of CMX is Brevilin A, a naturally occuring compound that has been shown to have an inhibitory effect on the JAK pathway. A simple internet search will yield many published studies on Brevilin A and JAK inhibition. Below is a look at the D.Nature pipeline; note that all three alopecia candidates are the same compound, albeit in different strength concentrations according to D.Nature CEO Paul Hong.

I will be following up with Mr. Hong to receive more information about the latest trial and formulation of CMX. For more background on CMX and a few examples of its hair growth effect from trials and users, take a look at theCMX/Anacell PDF Presentation.这种化合物的一个有趣的方面,而不是成功ly explored yet as far I know, is the potential of appyling it to alopecia areata. From my review of the PDF, I consider this product to have a mild hair growth effect in AGA. Like with many new drugs, I contemplate the potential of using them in combination with other treatments. For example, there was recently another product developed in Korea, CosmeRNA, which showed a mild, but documented effect on hair growth. The prospect of using these two new products together, with virtually no potential side effects, could certainly be interesting to members of this audience who are looking for drug free options.
Nanoparticles To Deliver Hair Growth Drugs (4/24/22)
If you’re one of the few who just checks the Updates page and is not subscribed to email updates when a new article is released, first of all please consider subscribing to email updates in the sidebar or bottom of page on mobile browsing. Secondly, head to the Articles page to read about two new interesting companies, Follicle Pharma and ProTransit Nanotherapy, who are using advanced delivery technologies to improve the outcome of hair growth drugs and prevent the drugs from substantially entering into the bloodstream.
Hope Medicine Shares Clinical Trial Design (4/12/22)
The phase 1 trial of Hope Medicine’s HMI-115 in androgenic alopecia is now listed on theClinicalTrials.govsite. While the trial is listed as “not yet recruiting”, there is still useful information shared. For starters, the trial will include both men and women – a detail that will be appreciated by many readers. Further, the trial duration will be 6 months with participants receiving a dosage injection once every two weeks. I don’t believe many people were expecting HMI-115 to be delivered via injection. The number of estimated participants is quite low, only 20. As of now, it appears that the only site location will be in Australia. Finally, the trial is expected to begin dosing patients by the end of the month, April 2022.
Epibiotech Selected By Korean Government For Areata Drug Development (4/6/22)
Hair Growth R&D powerhouse Epibiotech recently announced positive news for its alopecia areata pipeline candidate. EPI-005, an antibody therapy, was selected as a “new drug expansion research project” by the the “National New Drug Development Foundation” of Korea. The initiative is a government sponsored project which will support the development of EPI-005 as a therapy for alopecia areata. A Koreannews articlementions that Epibiotech is expected to receive support for up to two years for the development of the antibody. EPI-005 is described as a therapy which neutralizes the key inflammatory protein which causes alopecia areata. At this time, EPI-005 is proposed as an injectable treatment requiring dosages into the scalp once every 1-2 months.
Artificial Intelligence And New Androgen Inhibitor (3/29/22)
Two new companies working on hair growth were recently announced by Follicle Thought. Life Code AI is an artificial intelligence company working in various therapeutic areas including androgenetic alopecia, Aranda Pharma is a small biotech startup with a novel androgen receptor inhibitor in its pipeline. Head to the Articles page to read more about these two new interesting companies.
Interview With Ernesto Lujan PhD Of dNovo (3/25/22)
A new brief Q&A has been shared featuring Ernesto Lujan, founder of dNovo. It’s early days for his company with only so much that can be divulged at this time, but an interesting read to be sure. Head to the Articles page to see the new interview.
TechnoDerma Medicines Moves Into Phase 1B (3/22/22)
On February 28, 2022, TechnoDerma Medicines announced via press release the dosing of the first patient in its phase 1b trial of TDM-105795. The trial will be a 28 day dose-escalation study which is designed to find the upper safe dosing of TDM-105795 in a future phase 2 trial. The company statedin the releasethat it is likely that TDM-105795 “is able to activate dormant hair follicle stem cells and induce anagen (growth phase) in telogen (resting phase) hair follicles when binding to hair follicle cell target receptor proteins.”This phase 1b trial is estimated to conclude in October 2022.
Cassiopea/Cosmo Merger With Updated Website (3/15/22)
It appears that Cassiopea S.p.A. and Cosmo Pharmaceuticals have completed their re-merger, as the old Cassiopea website redirects toCosmopharma.comnow. On the linked Cosmo Pharma website, the Breezula page shares this quote in the second to last paragrpah, “discusisons are ongoing with the FDA and pending the sucessful outcome of these discussions we expect the commence the phase III trial in the second half of 2022.”The phase 3 trial of Breezula has been about two years in the making, it would be exciting news for many if the trial were to commence this year.
Innovation For Treatments (3/14/22)
Below is a tweet I wrote over the weekend which received a good response. It’s a sentiment that’s crossed my mind several times over the years as I have become better acquainted with the Follicle Thought audience. We’d all like to see a positive change. Please get in touch through the世界杯2022入围名单 page if you are interested in making a positive change.
Much too much “wait and see” attitude from investors, industry, and wealthy people when it comes to hair growth therapy development. Innovation requires taking chances with capital to create breakthroughs. Look at what’s being done in#longevitybiotech.#investors#crypto#aging
— Follicle Thought.com (Hair Growth Research) (@FollicleThought)March 12, 2022
siRNAgen Hints At 2022 Release For CosmeRNA (3/9/22)
siRNAgen/Bioneer Corp has been quietly making progress at launching their siRNA cosmeceutical in Europe over the past several months. Head to the Articles page for an update on the CosmeRNAarticlewhich is currently sticked to the top.
Dermaliq Article With Extra News (3/8/22)
Just before Kintor Pharma issued their press release last week, a new article featuring Dermaliq, Omega Therapeutics, and Rise Technology was published. Dermaliq is a new US based pharmaceutical company which is developing a prostaglandin f2alpha agonist drug for AGA. The new article is just below the stickied Kintor article on the main page.
Kintor Begins Dosing Patients In US Phase 2 Trial (3/1/22)
And just like that another Kintor trial is underway. This time it is a US phase 2 trial of the topical AR antagonist pyrilutamide in males with AGA. The trial will comprise around 120 male participants who will receive either pyrilutamide or placebo over the course of 6 months. Per today’spress release, the primary endpoint is the change in non-vellus hair count from baseline to 6 months in the treated group compared to placebo.
Dr. Farjo Talks HairClone Timing (2/26/22)
Dr. Bessam Farjo of the UK shared a recent interview he did with theNew York Social Diaryon Twitter today. Dr. Farjo’s commentary was shared in an article titled “Hair Cloning — the ultimate cure for baldness?” There is not much new or highly technical information in the piece (at least to this informed audience),however, Dr. Farjo does reiterate a timeline in which HairClone could begin offering treatments. The most recent perspective is that treatments could begin by the end of 2022 – early 2023.
Epibiotech Moves Towards Clinical Trial In 2022 (2/24/22)
On February 10, 2022 it was announced that Epibiotech and Linked Bio signed a business agreement for the purpose of advancing Epibiotech’s cell therapy EPI-001 into clinical trials. Linked Bio is a special consulting firm that specializes in preclinical and clinical trial project management. Epibiotech has called on Linked Bio’s services with the goal of obtaining an IND from the Korean Ministry of Food and Drug Safety in the second half of 2022.The news was announced in an article inMoney Today, a Korean media outlet.
Revivv Serum Released (2/22/22)
A new hair growth serum with formulas for men and women has been released to the public. The formulas have been tested in the office of Dr. Jeffrey Rapaport and presented on Follicle Thought with global and trichoscopic photography. Additionally, Follicle Thought has entered into an affiliation withRevivvfor the launch of this product. The main Articles page has all of the data available.
Almirall’s Finjuve To Be Marketed In Korea (2/14/22)
Boryung Pharmaceutical has entered into an agreement with Almirall to sell its topical finasteride formulation Finjuve in Korea. The pressannouncement是在2022年1月21日,但这篇文章吗not mention a timeline for the initiation of sales. A director at Boryung was quoted in the article to say that Finjuve maintains similar efficacy, while leading to a blood concentration around 1/100th of 1mg oral finasteride.
First Patient Dosed In New US Kintor GT20029 Trial (2/3/22)
2022 is absolutely on a roll. In the early morning of February 3, 2022, Kintor Pharmaceutical of China announced that the first patient has been dosed in a phase 1 trial of the drug GT20029 for AGA and acne vulgaris in the United States. GT20029 is a more advanced and newer type of anti-androgen drug than Kintor’s other candidate pyrilutamide. Check out the Articles page to get the link to the press release and bonus news about a pyrilutamide US phase 2 trial while the article isstickiedto the top.
Topical siRNA Treatment Could Be Sold As OTC Product In US (1/31/22)
siRNAgen Therapeutics is hoping to release a cosmeceutical hair growth product in the United States in 2022 or 2023. The company has completed two clinical trials of their CosmeRNA product in male and female pattern baldness which produced a positive result. Results from these trials were published in the Nature journal today. siRNAgen also says there is an avenue to develop their siRNA as a pharmaceutical treatment as well. Head to the Articles page to get the full scoop.
Junji Fukuda Of Yokohama University Establishes Company (1/28/22)
Another popular researcher in the field of hair follicle multiplication is making waves on the business side of things. Junji Fukuda PhD of Yokohama National University has apparently created a company “TrichoSeeds Co.” for the development of his hair follicle multiplication and mass production technology. Head to the Articles page to get more details on this development while the Fukuda Labarticleis stickied to the top.
Hope Medicine Gets Phase 2 Approval For HMI-115 In US (1/26/22)
More good news at the beginning of 2022. The long awaited phase 2 trial of Hope Medicine’s PRL receptor antibody treatment in AGA is now prepared to be launched. Many online enthusiasts had been awaiting news for this trial due to rumors about the drug’s impressive results in a small monkey trial. Head to the main Articles page to hear the latest on Home Medicine’s antibody drug HMI-115 and upcoming phase 2 trial in the US.
dNovo Announces Seed Round Financing (1/24/22)
On January 24, 2022, dNovo issued a press release to announce a successful round of fundraising from several Silicon Valley-associated VC firms and angel investors. dNovo was able to net $2.7M in their seed round, which is a significant raise for a hair growth biotech. This funding will allow dNovo to expand their development efforts into a more clinical direction. Today’spress releasealso hints at dNovo’s interest to secure an industrial partner to help really accelerate the development of their hair regeneration therapeutic.
Follica Shares New Poster With Results (1/19/22)
The Boston-based company Follica has broken a long period of silence by issuing apress releasetoday. The purpose of today’s release is to share a poster which Follica had recently presented at the Winter Clinical Dermatology Conference held in Koloa, Hawaii. The poster titled “Unmet Treatment Needs in AGA and Interest in a Novel Therapeutic Approach: Survey Results in >300 AGA Patients”displays data from a survey which Follica conducted with male participants ages 18-49 who have experienced hair loss. The purpose of the survey was to determine patient interest in Follica’s treatment program.
It turns out that around 74% of the participants (including a small % who were neutral) indicated that they would be likely to request Follica’s treatment based on photographic examples of previous results from the treatment. To view the Follica poster presentation which includes new photographic data of FOL-0054click here.
New Company dNovo Enters The Scene (1/18/22)
Around the start of the new year, a company which has been working under the radar since 2018 launched their website and announced themselves to the world. That company is dNovo of Northern California, USA. Harnessing the power of cellular reprogramming, an advanced new therapeutic technique, the company seeks to regrow hair follicles using stem cells. Head to the Articles page and dNovo’s website to get a full rundown of what the company has shared with us so far. 2022 is off to a good start.
Technoderma Completes Phase 1 And Prepares For Phase 1B (1/8/22)
On January 5, 2022, Technoderma Medicines announced the successful completion of its phase 1 trial for the drug TDM-105795 in which no adverse events were reported. Also announced was the initiation of recruitment for a phase 1b trial of TDM-105795 which is designed to be a dose escalation study. In this phase 1b trial, the goal is to identify the highest/safest dose of TDM-105795 to be used in a further phase 2 clinical trial. According to Technoderma’swebsite,第一阶段b审判将在一段时间内of only 28 days, meaning it is very likely that TDM-105795 will begin recruitment and begin dosing patients in a phase 2 trial later this year. Good news and progress from Technoderma Medicines.
Kintor Pharma Doses First Phase 3 Patient (1/1/22)
第一个男病人已经收到了ei的剂量ther pyrilutamide or placebo in China, according to a press release issued by Kintor Pharmaceutical today. This important announcement marks the second drug to reach a phase 3 trial for AGA since finasteride (SM04554 was the first). For all the details on the trial, head to the Articles page to read the updated Kintor Phase 3articlewhich is currently stickied to the top of the feed.
Nichinichi Pharmaceutical Co. Patent (1/1/22)
A pharmaceutical company from Japan which specializes in bacteria-based therapeutics has recently published an interesting patent related to hair growth and hair loss prevention. Unlike most patents, this one contains several before and after images of human subjects who apparently tested the product. At least one result shown would be considered extremely significant if it were proven to be authentic. Head over to the main Articles page to read more about this enigmatic patent from Nichinichi Pharmaceutical Co.
Replicel Terminates Shiseido’s RCH-01 License (12/21/21)
The saga between Replicel and Shiseido may not be ending anytime soon. Today, Replicel issued a press release announcing that they have terminated Shiseido’s license to commercialize RCH-01. Shisiedo happens to currently be in the middle of a clinical trial involving RCH-01 with plans to commercialize the treatment if successful. The Shiseido RCH-01 trial article is currently stickied to the top of the Articles page with a link to today’s press release.
Stemson To Present At Global Hair Loss Summit 2021 (12/8/21)
Stemson CEO Geoff Hamilton is scheduled to deliver the keynote address at the 2021 Global Hair Loss Summit titled “Developing Induced Pluripotent Stem Cell Technology to Engineer a De Novo Supply of Hair Follicles to Treat Hair Loss.”’ Hamilton be joining many other hair restoration professionals to give presentations at the Summit, which is tailored towards the hair transplantation industry. TheGHLS 2021is taking place December 11-12, 2021 and will be presented virtually; registration cost is listed at $499. It would be interesting to hear what Geoff says to say during his presentation, although, I would be surprised if anything distinctively different from his Follicle Thought interview will be shared.
Kintor’s Pyrilutamide Cleared For Phase 3 In China (11/24/21)
In perhaps record setting time, Kintor Pharmaceutical’s pyrilutamide drug has received clearance from the Chinese NMPA regulatory agency to begin a pivotal phase 3 trial in China. The trial is expected to begin in January 2022. Full detailshere.
Epibiotech Closer To Human Trial For Cell Therapy (11/20/21)
The most prominent hair growth research company in Asia and probably the most diverse hair research company in the world, Epibiotech of South Korea, recently completed an interview with Follicle Thought. Epibiotech’s research focus is the creation of an injectable dermal papilla cell therapy which can create significant hair growth/regrowth. They have been working on various methods to maintain the potency of dermal papilla cells when cultured outside the body. Ultimately, a clinical trial will validate whether the EPI-001 cell therapy performs better than previous industry attempts. Head to the main Articles page to read the Epibiotech 2021 Interview and hear more about their clinical trial plans.
Kintor Begins Female Phase 2 Trial In China (11/11/21)
The company that won’t stop making progress, Kintor Pharmaceutical, has announced the commencement of another phase 2 trial for pyrilutamide. This time it’s an all female study involving 160 participants and is taking place in China. The duration ofthis trialwill be 6 months, so we can expect topline results around late summer 2022. By the end of this year we are also expecting a full data release from Kintor’s successful phase 2 trial of pyrilutamie in males in China.
Almirall Topical Finasteride Approved In EU (11/8/21)
A press release issued on November 1, 2021 states that Hikma Pharmaceuticals of London, UK has entered into a license agreement with Almirall to market its topical finasteride formula “Finjuve”, previously known as ALM12845 and P-3074. According to therelease, Finjuve is already approved for use in Germany, Italy, Luxembourg, and Portugal. Hikma will now look to commercialize the topical drug in certain Middle Eastern and North African territories. Now that Europe has caught up to the Western world in terms of topical finasteride availability, hopefully this propels newer advancements in hair growth science globally.

University of Erlangen Discovers Hair Growth Molecule (10/31/21)
A team of researchers from the University of Erlangen in Germany have discovered a new molecule, specifically a soluble form of the CD83 protein, which they believe will be useful for hair growth. The team was awarded with the prestigious m4 Award from the Free State of Bavaria for their research. This award provides prize money that the team will use to form a new company called “MalliaBiotech” to further develop their hair growth protein. We will be following the progress ofthis storywhich has been making headlines in German news outlets over the weekend.
Hair Growth Companies Master List Article (10/22/21)
If you haven’t seen it yet, head to Articles page to checkout another landmark article titled “Androgenetic Alopecia Therapies Master List 2021.” There is now a whopping 40 individual companies/groups creating new legitimate hair growth technology. This amount of activity has never been seen in the industry before, we’re ready for a change.
Replicel Files For Arbitration Against Shiseido (10/18/21)
It has not been a secret that over the last several years Shiseido and Replicel have not been getting along perfectly. Back in 2013, the companies embarked on an agreement in which Shiseido paid Replicel around $4.2M CAD to gain a license to trial and commercialize the RCH-01 technology in Japan. As part of the agreement, Replicel was also supposed to conduct another clinical trial of RCH-01, but to this date they have not. Because of this, Shiseido claims that Replicel breached their end of the bargain, and thus, Shiseido has not shared any of its clinical data with Replicel. In its defense, Replicel maintains that it did not have the expected financial resources to complete a clinical trial for RCH-01 over the last 8 years. Now in 2021, Replicel has officially filed a没有tice of Arbitrationwith Shiseido and will undergo legal proceedings to bring the issue to resolution. In the meantime, Shiseido is still completing its 2nd clinical trial of RCH-01 involving repeat injections of the cell therapy.
Amplifica Progress And Potential (10/6/21)
I was lucky to get a useful response this week from Dr. William Rassman, an experienced hair transplant surgeon and founding member of Amplifica. For those unfamiliar, Amplifica is a biotech startup aimed at growing hair based on the discoveries of scientific founder Dr. Maksim Plikus. On September 15, 2020, the company issued its firstpress releaseintroducing the science of studying hairy moles/nevi to discover hair growth promoting molecules.This week, Dr. Rassman shared this response about the current progress of Amplifica:
“The human hairs growing in mice show that we have a powerful influence on kicking the Anagen Cycle on when compared to a control group. Sometime by the end of this year, wemaymake a decision to commence either limited clinical trials or an IRB study with a select group of doctors. I am very excited about the results I have seen and think that what we have has great promise.”
Realistically, because of the timeline involved this is some of the best news that we could have received. Dr. Rassman has been an ethical voice in hair surgery for quite some time and although he is part of the company, I believe his excitement comes with merit. We’re all very hopeful to see a formal human study started by February 2022.
New York Times Article On Hair Stem Cells (10/4/21)
The topic of hair loss research had made its way into another New York Times article. This time, the focus is astudyled by Dr. Rui Yi which was published inNature Agingtoday. Yi et al showed that as aging occurred in mice, stem cells residing in the bulge of the hair follicle began to migrate away from their home which led to the miniaturization of the hair follicle. The group identified two key genes which are necessary to keep follicle stem cells on their best behavior, i.e. remaining in the bulge region, Foxc1 and Nfatc1. There is no talk of translating this research into a clinical application withinthe NYT article, which is probably a more accurate way of reporting these things. Hopefully the attention from this article draws more interest towards further developed therapies within the industry.
Daewoong Pharmaceutical To Begin Finasteride Injection Trial (9/27/21)
The news is rolling in. It was announced today that Daewoong Pharmaceutical of Korea received approval to begin a phase 1 trial for its long lasting finasteride injection therapy in Australia. This is the technology that was developed by Inventage Labs of Korea which involves polymer-base microspheres which create a slow-release injectable formula of finasteride.IVL3001is the codename for the drug which can be delivered just once a month via injection. There is also a version of this drug which can last up to 3 months per injection, but it is not entirely clear which version is going into trials at this time.
Seoul Researchers Develop Hair Growth Peptide (9/26/21)
A team from the Seoul National University Hospital has published a recentstudytitled “Discovery of a transdermally deliverable pentapeptide for activating AdipoR1 to promote hair growth“在EMBO分子医学杂志上。的peptide of interest is called APN5 and mimics the action in which the adiponectin protein binds to its receptor. The research team, led by Profs. Chung Jin-ho and Lee Hyung-ho, tested the APN5 peptide in extracted human hair follicles in a petri dish and in mice studies. All testing confirmed that APN5 possesses a hair growth-promoting effect. A Korean newsarticlestates that APN5 was tested against 3% minoxidil in a mouse study, which is strange, however, Prof. Chung claims that APN5 has a superior effect over minoxidil. The article also mentions plans to begin clinical hair growth research with the peptide. Stay tuned for further updates on this work.

Stemson Therapeutics Exclusive (9/23/21)
In one of our proudest accomplishments to date, Follicle Thought is pleased to share an exclusive interview with Stemson Therapeutics’ CEO Geoff Hamilton. The interview is a transcription of a conversation which took place several weeks ago and provides a comprehensive profile of Stemson’s current progress and undertakings. This content could not be found anywhere else in the world right now; a national publication would not have captured half of the detail presentedhere.I hope you all enjoy it. Head to the Articles page to read about the latest at Stemson Therapeutics.
New Q&A With Dr. Kang-Yell Choi PTD-DBM Hair Researcher (9/14/21)
We’ve got some new information to share on the evolution of the PTD-DBM hair growth therapy which stemmed from research in the lab of Dr. K-Y Choi. The technology is now being developed as a small molecule which mimics the activity of the PTD-DBM peptide. Head to the main Article page to read a new mini-interviewwith Dr. Choiof the CK Regeon company while it is stickied to the top of the page.
Cassiopea Phase 2 Results In Females (9/11/21)
Yesterday, Cassiopea issued a press release sharing some details from the results of its phase 2 trial of clascoterone for the treatment of female androgenic alopecia. The study comprised 293 women aged 18-55 who received a twice daily dose of clascoterone or vehicle over a 6 month period.According tothe release“only the subgroup with women less than 30 years of age receiving twice daily application of 5% clascoterone solution showed statistically significant differences from baseline in TAHC at Month 6.”This would imply that the “complete” phase 2 trial itself did not reach statistical significance; it’s a way to share the news in a positive light.
It would be surprising if Cassiopea elects to continue its program for female androgenic alopecia after a phase 2 trial did not reach its endpoints.
Kintor Succeeds In Phase 2 Trial (9/8/21)
We are thankful to have Kintor. The company just can’t stop putting out press releases about new drugs, new trials, and now, successful trials in treating AGA. This latest news comes as Kintor has announced good news from its latest phase 2 trial of pyrilutamide or KX-826 in China. Head to the main Articles page for the link to the press release.
SM04554 Disappears From Biosplice’s Website (9/7/21)
Is this the end of SM04554 aka dalosirvat? Some readers brought to my attention this morning that SM04454 has been removed from Biosplice’spipeline page.There is no longer a mention of the drug or an androgenic alopecia program on the Biosplice (formerly Samumed) website. While there has been no official statement from Biosplice about the dissolution of its AGA program, this recent occurrence coupled with a long delay in communication following the completion of its phase 3trialof SM04554 in January are not promising signs. It’s possible that we have seen the last of SM04454. Stay tuned for any further clarification from the company.
New Companies Discussed Q3 2021 (9/1/21)
A new article has been published giving the spotlight for the first time to several new companies who are developing hair growth therapies. Companies include AddPharma of Korea, Novaliq in Germany, and the Rizn consumer product from Canada. Head to the main Articles page to get the full read.
Verteporfin Wound Healing Update (8/29/21)
I have a brief update on a topic that gained a lot of reader interest when first posted. The drug verteporfin which is on the market as an FDA-approved eye medication showed notable scarless wound healing results in a mouse model. This led to curiousity and discussion around the idea of trying the drug off-label in human wounds. I’ve received a response from the lead investigator of the verteporfin studies, Dr. Michael Longaker of Stanford, who had this to say:
“We are just finishing up large animal pig studies and then we will initiate human trials. We have filed intellectual property for both reduced scarring as well as hair follicle neogenesis. Anticipate human trials within 2 years.“
没有te that this does not prevent other doctors from trying verteporfin in a human patient sooner if they feel it’s a reasonable attempt. I will follow up with Dr. Longaker in the future about the results of the pig studies which are considered the gold standard of preclinical skin models.
HairClone Pushes Back Schedule (8/18/21)
我也e been meaning to share this update officially for a while, but have been putting it off because, well, it’s not awesome news. HairClone, the British company which launched the first ever equity-crowdfunding campaign for a hair growth treatment back in 2018, has had to push back their projected timeline for offering cultured dermal papilla cells in the UK. After receiving a few fortunate innovation grants from the UK government at the beginning of 2020, it appeared that physicians in the UK would be able to treat patients with HairClone cells by the end of 2021. Because of the common delays which have occurred over the last year and beyond, even 2021 will be too soon to see DP cell injections taking place. A recenttweetfrom HairClone reads as follows: “Hair follicle banking is already available via our clinical partners. Using these hairs to achieve hair rejuvenation will hopefully be in 2022“
At present it seems even 2022 is a hope. I wonder what everyone thinks about that crowdfund now?
New Public Company Omega TX Has Sights On SFRP1 (8/9/21)
Omega Therapeutics of Cambridge, MA completed their initial public offering (IPO) two weeks ago and is now publicly traded on Nasdaq. The company is developing a novel therapeutic platform called “Omega Epigenomic Programming”, which involves a new class of DNA-sequence-targeting, mRNA-encoded programmable epigenetic medicines. Alopecia happens to be a disease indication inOmega’s pipelinewith the SRFP1 gene specified as the target. Most will remember the SFRP1 protein was highlighted in research done at the University of Manchester in 2018.
Alopecia Areata Pipeline (8/9/21)
Last week, I published an article detailing a multitude of new companies working on treatments for alopecia areata. The majority of therapies involve JAK inhibitors, while other early-stage programs are utilizing peptides and siRNA technology. The article is intended to bring new hope and discussion to the patients and advocates of AA treatments.
Kintor’s GT20029 PROTAC Drug Doses First Patients (7/28/21)
The first few subjects participating in Kintor Pharmaceutical’s phase 1 clinical trial of GT20029 have received doses of GT20029 or placebo, according to a press release issued today.GT20029is an advanced type of androgen receptor degrader with potential benefits over 5AR blockers and androgen receptor antagonists. Earlier this month, Kintor announced that GT20029 had received INDs in China and the US to begin phase 1 trials for androgenetic alopecia and acne vulgaris.Today’s releasenotes that a phase 1 trial has begun in China. Based on their track record of rapid developments, we can expect a notice for a phase 1 initiation in the US soon, stay tuned.
Sustained-Release Finasteride Via Microarray Patch (7/21/21)
A new peer-reviewed study was conducted by researchers from Queen’s University in the UK and Hasanuddin University in Indonesia on the delivery of finasteride through microneedle array patches. The purpose of the research is to evaluate an alternative to oral administration of the drug, with an added benefit of sustained release. The attractiveness of this type of product is probably debatable among oral finasteride users, but it’s interesting nonetheless. The researchers concluded that adissolving and implantable microneedle array patchfor sustained release of finasteride could be useful for treating androgenetic alopecia as well as benign prostatic hyperplasia.
Stemson Attracts More Investment And Momentum (7/16/21)
A Series-A financing round netted Stemson Therapeutics another $15M this week. The company is getting the attention and resources it needs to develop the most sophisticated hair regeneration treatment the world has ever seen. Even their CEO Geoff Hamtilton knows how important this endeavor may be. The full scoop is on the Articles page.
Kintor Keeps Going (7/13/21)
从Kintor好消息时下雨,它倒。两个达ys after news of a US phase 2 trial for pyrilutamide was announced, Kintor Pharmaceutical has more good news to share regarding their more advanced drug for hair growth, GT20029. The news centers around a phase 1 trial coming soon to the US and Japanese phase 1 beginning immediately. The Articles page, where theGT20029story is currently stickied, has more details.
Pyrilutamide Moving Ahead On All Fronts (7/11/21)
Kintor Phatmaceutical’s most advanced AR antagonist drug for the treatment of androgenic alopecia is making significant progress in both the US and China. Today, it was announced that the FDA has approved a phase 2 trial of pyrilutamide in the US. There’s also important news brewing around Kintor’s phase 2 and upcoming phase 3 trial in China. Head to the main Articles page to read anupdated articleon Kintor while it is stickied.
Koehler Lab Hair-Bearing Skin Update (7/7/21)
More good news from Harvard. The lab of Dr. Karl Koehler has made some notable progress since we last heard from them a little over a year ago. In a recent response from Koehler, he revealed that his team is working on two research projects which could potentially lead to new therapies in the hair restoration game. Head to the main Articles page to read a quote from Koehler about his progress while the article is stickied to the top over the next several days.
David Sinclair & Harvard Trying To Solve It (6/28/21)
I recently had a fortunate interaction with world famous aging researcher, David Sinclair PhD of Harvard University. It turns out, his laboratory and other Harvard labs are doing extensive hair follicle restoration research. Head to the main Articles page to read the verbatim response from Sinclair about his current studies which could potentially lead to a hair growth solution for the masses someday.
Minoxidil Booster Study Published (6/18/21)
A long promised study on the Minoxiboost product aka SULT1A1 from Applied Biology was published in the Journal of Cosmetic Dermatology earlier this week on June 16th. The study titled “SULT1A1 (Minoxidil Sulfotransferase) Enzyme Booster Significantly Improves Response to Topical Minoxidil for Hair Regrowth”includes Drs. Jerry Shapiro, Rodney Sinclair, Rachita Dhurat, and Andy Goren as authors, among others.
As for the statistics that everyone is looking to hear: 24 males with an average of NW4 comprised the study. The subjects were divided into two groups of 12 men each. Both groups applied 5% minoxidil daily, one group also applied the SULT1A1 booster product and the other added a placebo control. In the end, the group using SULT1A1 with minoxidil showed a 75% hair regrowth response rate among participants (9 out of 12), whereas the group using minoxidil with placebo showed a 33% hair regrowth response rate among participants (4 out of 12). Thesmall studyshows efficacy for SULT1A1 improving hair growth response for minoxidil users.
mRNA Therapy For Treatment Of Hair Loss (6/16/21)
A company working on anti-aging medicine will pursue hair growth as one of its first therapeutic targets. Stemming from research done at Stanford University, Turn Biotechnologies harnesses a novel mRNA platform intended to revert human cells to a healthier, younger state. Head to the main Articles page to read more about the company and their lead candidate TRN-001 aimed at dermatology indications.
Young Man Shares About His Toupee (6/13/21)
I recently came across a video on social media about a male Tik Tok creator who is open about wearing a toupee and thought that the perspective would be appreciated by some of the audience. I considered embedding the video, however, ultimately I thought it would be best to simply link it. The video starts off a little goofy, but progresses into some worthwhile anecdotes from Jordan, the Tik Tok creator. Here’sthe linkto video from LADbible, and if you’re not able to access it that way you can search for a video from them posted on June 10th 2021 titled “What Life Is Like With A Male Toupee.” Jordan has a positive and carefree attitude about his decision to wear a hair system and I thought that overall, his disposition about his hair would be valuable to share here. There was also aBuzzfeed articlewritten about Jordan recently.
OliX PharmaceuticalsPartners To Further Hair Program (6/10/21)
It’s always a good sign when companies who are working on hair therapies enter into partnerships to advance their therapies. Yesterday, on June 9, 2021, the Korean biotech company OliX Pharmaceuticals announced its new partnership with LGC Biosearch Technologies of England. Under the agreement, LGC Biosearch will produce OliX’s OLX104C silencing RNA therapy for upcoming preclinical and clinical trials. OLX104C is a topically administered therapy. OliX believes that their therapy may be an effective new treatment for androgenic alopecia with reduced systemic side effects. Thepress releasementions that a clinical trial for OLX104C is expected by the end of 2022.
Farewell, Histogen (6/3/21)
In an unsurprising turn of events, Histogen announced today that they are “suspending” the development of HST-001. Although suspension is a term used to describe temporary periods of inactivity, we can be all but sure that this is the关闭chapterfor HST-001 aka HSC which we have been hearing about over the last 10 years. The decision comes after disappointingphase 2a trial resultswere announced earlier this year in February. And so, it appears that the epic drama known as “Hair Stimulating Complex” finally comes to a close. In retrospect, the most confusing part about all of this is the superb data that the product exhibited in its early years. In 2019, I wrote anarticledetailing the wild ride that Histogen took over the past 12 years which included lawsuits and multiple year delays.
It certainly has been a long, strange trip. Good luck Histogen.
Epibiotech (Stemore) Gets Closer To Clinical Trials (6/2/21)
One of the most dedicated hair growth R&D companies in the world,Epibiotech(formerly known as Stemore) of South Korea, was recently featured in a Money Todayarticleto announce the completion of their new GMP facility in South Korea. This facility will serve as a licensed space where the company can culture dermal papillae and other cells to be used as hair growth treatments. Importantly, in the article, Epibiotech CEO Sung Jong-hyeok states that he “expects the completion and submission of the non-clinical trial stage and clinical trial plan(for EPI-001)this year.” EPI-001 is Epibiotech’s lead therapy candidate which involves a proprietary process of culturing and multiplying dermal papilla cells for injection. This would likely point to a clinical trial for EPI-001beginning in Q1 2022. What’s neat about EPI-001 is that the article mentions that only 2-3 hair follicles are needed from a patient to create the therapy. Source for this news foundhere.
TechonoDerma进入试验& Aneira增加资本(5/25/21)
一个新行业从中国球员,TechnoDerma读出cines Inc., has begun a phase 1 clinical trial for the treatment of male AGA. The trial is actually being carried out by a contract research organization based in California, USA and is still recruiting participants. Other industry news includes the startup Aneira Pharma raising $25 million to start their own trials for AGA. Aneira has developed an undisclosed formulation of hair growth-promoting molecules which purportedly can regrow as well as darken hair. For more information on these news items head over to the main page to read the latest article.
Replicel Seeks To Improve Inductivity for RCH-01 (5/18/21)
Last week, Replicel issued a press release announcing the second stage of its research collaboration with the University of British Columbia for the purpose of creating a hair follicle gene expression “map.” According tothe release, the initial research done at UBC in 2017 “examined multiple cell types from human hair follicles to identify differences, and similarities, in gene and protein expression with the goal of finding markers of interest that would allow potential optimization in target cell isolation from the initial patient tissue biopsy as well as post-manufacturing final product formulation…”没有w, after recently securing morefunding与MainPointe制药、Replicel is able to fund the second wave of research at UBC which will seek to confirm the presence of the more potent “hair growth inducing” cells in hair follicles and develop techniques for extracting these cells efficiently so they can cultured and expanded.
In simple terms, Replicel is looking to identify the most potent and inductive cells within the dermal sheath cup of a hair follicle in order to precisely extract them and utilize them in the culturing process of RCH-01. The overall goal is to improve the hair growth capability of RCH-01. I have learned from a scientist at Replicel that improving the inductivity of RCH-01 in this manner would not require them to restart any clinical trial process as long as the cells are still derived from the dermal sheath cup. We wish them well.
Verteporfin’s Potential For Hair Transplants (5/11/21)
A new article has been published on research from Stanford University involving the drug verteporfin, which is an FDA approved drug for eye diseases. It turns out, the drug may have important applications for wound healing, and thus, it may have important applications for hair transplant surgery. As an FDA approved drug, it is possible that doctors may begin experimenting with verteporfin in wound healing cases per their own discretion. Head to the Articles page for more information on this fascinating discovery.
Hope Medicine Raises $56M For Global Trials (5/7/21)
And just like that, when one company bows out another (perhaps more formidable) candidate steps forward. A fan favorite, Hope Medicine of China, has recently completed a Series B round of financing in which $56M was raised for the company. From thepress releaseon May 6, Hope Med’s CEO Rui-Ping Xiao noted:“This round of financing will provide strong support for the company’s upcoming MRCT international phase II clinical studies and commercial product development.”The phase 2 clinical studies will be for HMI-115, Hope’s PRL receptor antibody drug which has shownpromising hair growthresults in a preclinical trial involving monkeys. According to the press release, the phase 2 trials will be launched “soon.” We can likely expect them to commence in Q3 2021.
Follicum Bids Farewell (5/7/21)
Results have come in from Follicum’s most recent phase 2a trial of FOL-005 in male AGA. The peptide drug was able to increase hair growth, but not quite enough to show a clear improvement over placebo. Head to the Articles page to read the full details and hear what’s next for Follicum.
Ultimate Guide Chart Update (5/4/21)

Our flagship article, The Ultimate Guide to Hair Regeneration, has been updated with a new pipeline chart which represents the most promising and advanced hair growth treatments in the industry. Theentire articlewill also continue to be updated this week.
HaplnScience & Rise Technology New Companies (4/21/21)
一个新的文章已经发表了两个新的companies in the hair growth game. One of the companies, Rise Technology, is a spin-out of the University of Wisconsin at Madison based on Dr. Wang’s research using electrical stimulation devices which fit into baseball caps. The other company, HaplnScience, is taking a completely new approach to hair regeneration by targeting a protein that may have a significant anti-aging effect on skin and hair follicles. Head to the main Articles page to read more.
Kintor Pharmaceutical Prepares For New Androgen Drug Trials (4/15/21)
It was announced that on April 14, 2021, the NMPA of China (equivalent to US FDA) approved the investigational new drug application (IND)for GT20029, an androgen receptor degrader, held by Kintor Pharmaceutical of China. The IND allows Kintor to begin human trials for androgenic alopecia and acne vulgaris in China.
In today’spress release, Kintor mentioned that they plan to begin recruiting patients for a phase 1 trial with a topically formulation of GT20029 around August/September 2021 for AGA. For more background information on the drug, check out arecent article on GT20029.
Hyundai Pharm Releases OTC Product Dexnoxyl (4/12/21)
更多的头发生长,related news has emerged from the Koreabiomed media outlet. Today, it was announced that the Hyundai Pharmaceutical company of South Korea hasreleased a new “OTC drug”Dexpanthenol in a pill form. Dexpanthenol is a derivative of panthenol, which is an analog of vitamin B5. You’ve likely been noticing panthenol or pantothenic acid as a main ingredient in your shampoo for many years, it’s widely known to have an affinity with hair health. At this time, there has been no data released by Hyundia Pharm on the Dexnoxyl product which leaves things unclear as to what a vitamin-b related pill could do as a treatment for hair loss, but the fact that a real pharmaceutical company has put this out is interesting.
New Korean Player Inscobee Patents Stem Cell Technology (4/9/21)
It would seem as though South Korea is one of the most active countries in the world when it comes to hair growth research, and they may actually be #1. Today, news was published in Korea aroundInscobee‘s patent using implanted stem cells to promote hair growth. According to Koreabiomed, the company has been granted a patent in the US for their “bioimplant” which will lead to a pre-clinical trial later this year.The description of the technology from Koreabiomed article is fairly vague, though, the approach sounds innovative. From thearticle:
“The company’s technology showed improvement in hair loss and enhanced the growth by implanting stem cells cultured under specific conditions into the body through capsules. Stem cells inserted in the capsule are designed to improve only hair growth without side effects.”
It sounds as if there is some sort of nano-encapsulation technology involved which may be used to preserve the stem cells or keep a mixture of cells intact. This may very well be a “hair cloning” type of therapy where a concoction of hair/skin stem cells are cultured and implanted back into the skin to form new hair follicles. In time, we will hopefully find out more about this approach.
Topical Finasteride Is Now Easily Accessible In The US (4/1/21)
My latest article covers two telemedicine startups in the US which are offering topical finasteride prescriptions to patients following a brief online consultation. Topical finasteride has been a topic of interest to some readers for a long time and is by far the preferred route for finasteride administration in my opinion. Now, it’s available without leaving your living room. Head over to the Articles page to learn more about the Strut and Happy Head companies.
Eli Lilly/Incyte Announce Positive Result In Phase 3 For Alopecia Areata (3/23/21)
This news made headlines a few weeks ago when Eli Lilly announced that its JAK inhibitor drug baricitinib showed astatistically significant increasein hair growth over placebo in both the low-dose and high-dose groups in a phase 3 trial for alopecia areata. Although JAK inhibitors are already being prescribed off-label for the treatment of alopecia areata, this recent milestone by Lilly brings their company the closest to having an FDA approved treatment for the auto-immune disease.
尽管试验中结果是积极的,但基于“增大化现实”技术e some general concerns over using JAK inhibitors such as baricitinib to treat AA due to potential side effects, according to the linked article. Lilly is among two other pharmaceutical companies (Concert and Pfizer) who are in late stage trials using JAK drugs for AA. Despite reporting a successful outcome from a phase 3 trial, Lilly is currently embarking on anadditional phase 3 trialfor baricitinib in alopecia areata before it would seek approval for the drug.
New Companies Coming In Hot (3/14/21)
Head over to the main Articles page to read about Topadur Pharma and Eirion Therapeutics, two companies with new therapies for AGA in their pipelines. Eirion even has a bonus therapy for gray hair reversal as well and just secured a $40M investment! The hair therapeutic industry continues to grow and grow, which is a great thing. ?
Tsuji Rolls Out Hair Growth Ingredient With Adjuvant Cosmetics (3/4/21)
Perhaps the world’s most famous hair cloning researcher Dr. Takashi Tsuji recently announced that work done in his lab has lead to the discovery of a new hair growth ingredient which is being commercialized in partnership with the Adjuvant Cosmetics company of Japan. The ingredient which is described as a functional peptide will be sold under the new brand name Kaisu. Head to the main Articles page for more details on this discovery.
An In-Vitro Model For Drug Screening Using Only DP Cells (3/1/21)
Researchers from the Institute of Biotechnology and Nanotechonlogy (IBN) in Singapore have developed a novel system for screening the effects of potential hair growth drugs using 3D cultured dermal papilla cells.Lead author of the study, Dr. Andrew Wan, contacted me recently to share that hispaper had finally been acceptedand offered to answer any questions I might have. It will surprise the readers to know that I had been in contact with Dr. Wan for about 4 years hoping to share more about his study after it published; at some point over the years we fell out of contact when it seemed as though the paper would not be published after all. The implications of this work have changed since I was first in contact with Dr. Wan as he is no longer working to progress the project and has moved into food sciences. He does tell me though, “I hope that someone else would be able to develop our work further based on what we have published.”
This work is interesting and the short-written study by Wan et al sheds light on the techniques that scientists are using to engineer and study lab-grown hair follicle organoids. Below is a neat depiction of the data obtained from the 3D cultured dermal papilla spheroids created by Dr. Wan and his team. Three hair growth promoting drugs (minoxidl, CsA, and bimatoprost) and one hair growth inhibiting drug (dexamethsone) were evaluated by observing the upregulation and downregulation of known anagen and catagen genes after the drugs were added to the DP microtissues. For example, we can see that minoxidl has a strong upregulation effect on VEGF, while bimatoprost has a significant effect on fibroblast growth factor 7. Dexamethasone, the hair inhibiting drug, shows an upregulation for catagen genes while downregulating the known anagen genes.

In short, this model can be used to screen the upregulation/downregulation effect of new drugs in agagen or catagen genes. Please feel free to share this work with all whom you feel may benefit from it.
Triple Hair TH16 Release Update + Applied Biology Tidbit (2/25/21)
The interesting product TH16 from the Triple Hair company is set to be released in early 2021. Originally, it was slated for a Q1 2021 release, but there has now been a slight delay announced by the company. Theall-natural blend from Triple Hairis said to contain melatonin and resveratrol and produced favorable results in a comparative trial against minoxidil 5%. The “Look Ahead to Hair Growth Therapies in 2021” article is now stickied to the top of the Articles page with more details on the TH16 release and distribution plans.
Also, in response to the readers who have been asking for an update regarding the Applied Biology minoxidil booster product release and the new clinical studies it has undergone, I have heard from Dr. Goren of AB that an official update will be announced soon. This was as all he would provide at the moment, so, I think we can expect some official news and data within 2 weeks.
Histogen Misses Statistical Significance (2/18/21)
In the latest clinical trial of HST-001 aka HSC, Histogen did not deliver the results we were looking to hear. After 26 weeks of observation, patients receiving injections of HST-001 did not show a statistically significant increase in total hair count compared to patients who received placebo injections. The news is not optimal, but somehow this is apparently not the end of the road for HSC. Head to the Articles page for the full story.
New RNAi Therapy Announces Preclinical Results (2/11/21)
New industry playerOliX Pharmaceuticalsof South Korea issued a press release earlier this week announcing their positive preclinical results in mice. In the study, topically administered OLX104C, an RNA interfering technology, outperformed the hair regrowth effect of topical flutamide and placebo in mice who had previously received DHT injections to suppress hair growth.

In theirpress release, OliX CEO Dr. Dong Ki Lee shared the following statements:“Due to topical administration, our investigational therapy acts directly on the affected area and is rapidly degraded upon exposure to the blood, so it’s free from major systemic side effects such as sexual dysfunction caused by conventional hair loss therapeutics. Building on our experience evaluating RNAi therapeutics for skin diseases, we plan to initiate clinical trials of OLX104C by 2022.”Hopefully the timeline to human trials will be as swift as possible for this interesting new therapy.
Tsuji Stem Cell Study Published (2/11/21)
It turns out that the news from Dr. Tsuji’s team was not about an impending clinical trial, but rather a new important study on the culturing of hair follicle epithelial stem cells for hair cloning. Head to the main Articles page for the full scoop. Hopefully, Dr. Tsuji will soon land an industry partnet to get humans trials going.
News From Riken Coming Wednesday (2/8/21)
In an unexpected development, the Riken Institute of Japan which houses the laboratory of world renowned follicle researcher Dr. Takashi Tsuji, has announced that they have made an important development which could lead to the clinical administration of their hair follicle cloning technique. From the company’sTwitter account(translated):“We have succeeded in developing a new technology that will lead to hair follicle regenerative medicine. The results will be published from 19:00 (Japan time) on February 10, 2021.”
This is an extremely fascinating development and you can be sure that Follicle Thought will be reporting on the announcement Wednesday. For clarity, the official Rikenwebsitehas created a 2021 update which verifies the legitimacy of the new Riken Twitter account. Let’s hope for some amazing news this week. ?
Kintor Creates New Advanced Androgen Receptor Degrader (2/1/21)
Head over to the main Articles page to read about GT20029, an advanced new form of anti-androgen called a PROTAC which is soon to be trialed for hair growth and acne in China and the US. This now makes two interesting androgen receptor-based drugs in Kintor Pharmaceuticals pipeline for hair growth.
Follicum Completes Phase 2a Study With Topical FOL-005 (2/1/21)
On January 28, 2021 Follicum announced vianews releasethat all participants have completed their dosing schedules and have visited the clinic for the final time in the latest phase 2a study of Follicum’s FOL-005 topical peptide. The enrollment for the study was just over 200 male subjects – a robust pool to get an accurate idea of efficacy from. CEO Jan Alenfall noted that only one patient withdrew from the study due to complications related to covid. Results from this important trial are still expected to be presented in Spring 2021. Be on the lookout in early March for the verdict on topical FOL-005.
Pelage Pharmaceuticals Making Progress (1/20/21)
I have a brief, yet, rare update to share regarding Pelage Pharmaceuticals, the UCLA spinout company which is developing drugs to activate hair follicle stem cells for hair growth. Since the time a press release was published announcing the inception of Pelage Pharmaceuticals inMay 2019there has literally been no available news on Pelage Pharma; no website, no interviews, no sign of activity, nothing.
This week I managed to get a response from Pelage Pharma founder Dr. Bill Lowry who is also a professor of molecular biology at UCLA. Regarding the current activities and plans ahead for Pelage, Dr. Lowry told me he could not disclose much at this time (this is likely due to the affiliation with Allergan who is an investor in the company), but he did tell me “happy to saywe are moving forward with urgency!” Typically, this wouldn’t be a breaking news story, but, as I’ve mentioned there hasn’t been a peep about Pelage over the last 19 months. Now, we can verify that the company is indeed active and making progress. I also appreciate the fact that Dr. Lowry showed enthusiasm and urgency when talking about his company’s drug development efforts.
CarthroniX To Develop Stem Cell Activating Drugs For Hair (1/14/21)
CarthroniX新文章已经发表,一个interesting biotech startup out of California which is developing small molecule therapies for tissue regeneration. One of CarthroniX’s interests is hair growth and they have previously published a paper on using specific molecules to stimulate lactate production which in turn activated hair follicle stem cells and the hair growth cycle. Read more about CarthroniX and their lead candidate RCGD423 on the articles page now.
Preview Of 2021 Milestones For Hair Growth Companies (12/30/20)
If you have not yet made it a habit of checking out the latest articles on Follicle Thought, please go bookmark theArticlespage in your browser for quick, convenient access. The latest installment on the Articles page is a synopsis of the most relevant company milestones which are planned for 2021. Samumed, Triple Hair, Kintor Pharma, and many more make noteworthy appearances on the list.
RiverTown Therapeutics Shuts Down (12/16/20)
This is some of my least favorite news to share, but it is necessary in order to bring closure and clarity to situations. After years of seeking funding to further their drug RT1640 in clinical trials without success, the company has decided to call it quits. Back in 2016, Dr. David Weinstein, founded of RiverTown Therapeutics, shared some impressivephoto resultsfrom his drug for the first time on Follicle Thought. The company was also fortunate enough to be the lead feature in aNew Yorker Magazinearticle on potential cures for baldness. However, the notoriety was to no avail, and a phase 2 did not come to frution for RiverTown. Below is an example of a long term result of RT1640 on a previously empty crown.

Follica Announces Peer-Reviewed Study For Females (12/9/20)
Today, Follica issued a press release announcing the completion of a pilot study treating female pattern hair loss with its proprietary microneedling device and topical drug. Theresultsof the study were published in theInternational Journal of Women’s Dermatology.According to the press release, 11 women with mild to moderate androgenic alopecia underwent 6 treatments with the Follica microneedling device and applied topical minoxidil on days when not receiving treatment. At the conclusion of the study, 10 out of 11 women reported self-perceived improvements in hair growth and all 11 women improved on their physician graded Sinclair scores (appearance of hair growth/health). Below is a photo result published in the peer-reviewed study.
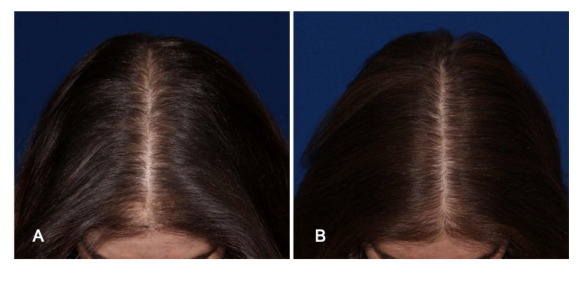
Thepress releasealso mentions that Follica is planning to initiate its phase 3 study for male androgenic alopecia in 2021.
Histogen Announces Preliminary Results From 2020 Study (12/1/20)
Per a company press release from this morning, Histogen has announced preliminary results from its phase 1b/2a trial for men with androgenic alopecia which began earlier this year. No statistical data was shared inthe release患者除了提到HSC demonstrated “separation” in total hair count from patients who received placebo injections. Histogen CEO Richard Pascoe states that the company is looking forward to reporting the full results after a week 26 assessment has been completed by all patients in early 2021. Let’s hope that a statistically significant result is found over the next 2 months.
Gene Therapy To Keep Hair Growing In The Works (11/30/20)
An interesting new article on the Moogene company of South Korea has just been published. Moogene is using CRISPR gene-editing technology to make hair follicle dermal papilla cells immune to DHT. Head over to the Articles page to read more.
NewDermal Papilla Cell Induction Research (11/24/20)
Researchers in China led by a group from Hunan University have published an interesting new study on the process of inducing dermal fibroblast cells into dermal papilla-like cells. The study is titled “Induction of dermal fibroblasts into dermal papilla cell-like cells in hydrogel microcapsules for enhanced hair follicle regeneration”and will appear in the December issue of theApplied Materials Todayjournal.
In the study, the team demonstrated that human dermal fibroblasts can be induced into dermal papilla-like cells through alginate-poly-L-lysine-alginate (APA) encapsulation.
The image above displays the process of encapsulating the human dermal fibroblasts which leads to gene upregulation of hair promoting factors, then, the addition of epidermal cells before being injected into mice which displayed hair regeneration. The authors of the study claimed that this method can be used as a novel source of DPC cells and may circumvent the bottleneck in DPC-based hair follicle regeneration. The “bottleneck” that the authors are referring to is the tendency for dermal papilla cells to lose their genomic profile (aka hair growth effect) after a number of passages in a 2D culture setting. Look for an update from this research soon on Follicle Thought.
HairClone Brief Check-In (11/18/20)
I happened to get in touch with HairClone CEO Paul Kemp earlier this week and during our exchange Dr. Kemp provided a brief status update on HairClone’s current progress and development. Please see his commentary below:
Stemson Planning To Move Research Into The UK (11/12/20)
An interesting development has been announced by Stemson Therapeutics, a company based in Southern California focused on developing hair regeneration therapies. In anews releaseshared on November 10, 2020, Stemson announced that they are targeting the UK as a location to further their R&D and potentially conduct their first human trial in. Perhaps the most interesting facet of this news is the fact that we are familiar with the UK having a unique regulatory allowance referred to as “Specials.” In the case of Specials, physicians are able to prescribe and administer treatments which have not gone through a full clinical trial process to patients if said treatments are determined to be reasonably safe and are filling a need where other treatment options do not yet exist. Multipled dermal papilla cell injections may be an example of this. The one catch is that companies who develop the up-and-coming treatments are not allowed to market their treatment to patients and the decision to attempt the treatment must solely come from the physician and then the patient.
It is a conceivable notion that Stemson may be interested in utilizing the Specials regulation as a way to test and fine-tune its hair regeneration therapy before conducting a full-fledged clinical trial in the UK. This announcement comes on the heels of a $7.5M investment that Stemson received from Allergan Aesthetics and Fortunis Capital, which is an investment firm based in the UK. Geoff Hamilton, CEO of Stemson, shared this statement regarding the potentials of expanding into Great Britain: “The UK is on our short list with its world class scientific talent and infrastructure, and I know Fortunis Capital certainly believe we will find full support for this level of scientific innovation in the UK.”?Interesting stuff going on here.
More Studies Coming From SULT1A1 Product (11/4/20)
我也e received some updated information from Dr. Goren of Applied Biology regarding the SULT1A1 product and its use by men, and those who are already responders to minoxidil. Head to the main Articles page to get the scoop. As it turns out, Applied Biology is conducting at least 2 new human studies with SULT1A1 to get answers to important questions.
Minoxidil Boosting Product Planned For January (10/23/20)
The sulfotransferase enzyme-increasing product from Applied Biology is planned for a market launch in January 2021. Follicle Thought had an exclusive interview with Applied Biology earlier in 2020 which stated that the product would likely be released in 2020, however, it seems that an expected delay this year has pushed the release to January next year. Look for exclusive updates about the product’s release, including a link to its new website, on the Articles page soon.
Epiregulin Protein Identified As Hair Promoter (10/19/20)
韩国延世大学的研究人员d by Nahyun Choi PhD have identified yet another key protein which could be developed into a new therapy for hair loss. The latest study on the epiregulin protein (EREG) was published as the cover paper of the September 2020 print version ofCell Proliferation.In the study, it was found that EREG has a positive effect on the hair growth cycle by activating the phospho-EGF receptor and the ErbB4 antibody in epidermal cells. Choi, who is also a lead researcher at the Stemore company, told the Korean media siteMoney Todaythat epiregulin is “expected to be developed as a cosmetic material as well as a hair loss treatment agent (drug).”
Anti-Aging Company Seeks Investors (10/18/20)
One of the most exciting companies in the aging reversal-treatment industry, Yuvan Research Inc., has recently opened their SAFE investment round and is seeking to connect with investors. The lead candidate therapy of Yuvan Research, called “Elixir”, is a fraction of young blood plasma which has shown to reverse biological age up to 50% in preclinical studies. Top scientists in the anti-aging industry, such as David Sinclair, have beenvery impressedwith this research so far. The company also has a proprietary over-the-counter topical gel in the works which is being tested for wrinkles and may also benefit hair growth pending further studies. If you are anaccredited investoror VC and would like to get in touch with Yuvan Research please reach out through the世界杯2022入围名单 form.
Shiseido Begins Second RCH-01 Clinical Trial (10/14/20)
In a very a surprising development, Shiseido has begun a second clinical trial of Replicel’s RCH-01 dermal sheath cup cell therapy. As they alluded to following the release of the first trial’s results, Shiseido will be testing the efficacy of RCH-01 after repeated doses this time around. The officialclinical trialpage does not mention how many doses will be given per patient, however, we do know that the trial will consist of 36 participants, both male and female, and will last for a duration of 12 months. Tokyo Medical University Hospital will conduct the trial, and as of today the trial is still recruiting patients. Inclusion criteria is a NW 3 degree of hair loss for men and a degree of 3 – 6 on the Shiseido scale for women.
It would be very interesting if Shiseido ends up surprising everyone by bringing forth a successful treatment after the completion of this trial. Stay tuned for updates.
Breezula Female Phase 2 & Follicum Phase 2 Trials Complete Enrollment (10/12/20)
Two European companies developing topical hair growth medications have filled up their patient rosters for their respective phase 2 trials last week. Cassiopea SpA announced viapress releaseOn October 8th, 2020 that 293 females between the ages of 18-55 have been recruited to participate in a 6 month study to test different concentrations of clascoterone (one of them is the standard 7.5% Breezula concentration) against minoixidl 2% and placebo. Results are expected in Q2 2021 and this should be one of the most interesting studies for all female patients seeking new treatments to grow hair.
Alsoannouncedon October 8th, Follicum has completed the enrollment of 200+ male patients who will be administering different concentrations of the peptide FOL-005 or placebo once daily over a period of four months. The last patient enrolled is scheduled to complete the regimen by the end of January 2021. Results from the trial are expected around the end of Q1 2021. The first half of 2021 should have some important news to share for the hair seeking community.
Kintor Pharmaceuticals Officially Pushing For Phase 3 In 2021 (10/8/20)
这是最快的一个临床试验processes in hair growth industry history, Kintor is aiming to go from phase 1b in 2020 to phase 3 in 2021 for their drug pyrilutamide. Head over to the main Articles page to read the full scoop.
Histogen Trial Is Completed And Data Comes Next (10/5/20)
Today, Histogen announced via press release that every patient in its phase 1b/2a trial for androgenic alopecia have received all of their injections. All that’s left are two more return visits for patients to take photographs and complete other routine health tests. Histogen is on track to present the data by theend of this year, most likely in December. The next two months will be extremely important for Histogen who is looking to finally partner the HSC program (think companies like Allergan) and make some money on a decade + long excursion.
Remember The Krox20 Research at UT Southwestern? (10/4/20)
Back in May 2017 media headlines were ablaze with the latest “cure for baldness discovery”, which came from the University of Texas Southwestern and was led by Dr. Lu Le. The discovery was centered around two proteins; one of the proteins, Krox20, was deemed essential for hair follicle formation, and the other, stem cell factor, was necessary for hair pigmentation. It caused a bit of excitement in the online hair research circuit, but most of us knew it would years before this discovery could be shaped into a practical application, if ever. One promising sign from this news was Dr. Le’sinitial interestin pursuing a topical medication to actualize his discovery.
I reached out to Dr. Lu Le recently to ask if he was still pursuing the Krox20/SCF research as potential treatments for hair loss and hair graying. I was surprised to get this quick response from him as follows: “Yes, we are expanding our research on those areas. Will send you our publications once they got accepted.”
We can tell from Dr. Le’s response that he has in fact been continuing his research on the Krox20 gene’s involvement in hair growth and is potentially doing preclinical work using a topical gene therapy. It’s not always the easiest to predict when peer-reviewed studies will be released as there are many variables when it comes to the peer-review process, but it seems likely that a paper would surface before Q2 2021. Be on the lookout for a potentially very important new Krox20 study being published.
Inventage Lab Prepares Two Versions Of Slow Release Finasteride For Trials (9/28/20)
According to the Korea Biomedical Review, Inventage Lab of Korea has secured a strategic partnership with Daewoong Pharmaceutical to bring two versions of finasteride encapsulated in a special delivery system to market. The two pipeline candidatesIVL3001 and IVL3002utilize Inventage’s Involucrin Precision Particle Fabrication Microsphere technology which allows the drug to be delivered over a sustained period of time. In these cases, IVL3001 delivers a month long supply of finasteride while IVL3002 packs 3 months worth of finasteride per dose. Human trials for IVL3001 are set to begin in Q1 2021.
Artifical Intelligence GANs Find Hair Growth Drug Candidate (9/23/20)
A newer type of neural network called a GAN or “generative adversarial network” is being introduced to the pharmaceutical industry. This type of AI application is said to be capable of shrinking the timeline of the typical drug discovery process from years down to weeks. The good news is a company using GANs to discover new drugs have found a promising molecule for the treatment of hair loss. Head over to the main page to learn more about the company Insilico Medicine and their amazing technology.
Well Known Researcher Spins Out Startup Company – Interview (9/18/20)
(Recent interview with Maksim Plikus, however he does not specifically mention his new company in the interview, it’s mostly technical stuff.)
Dr. Maksim Plikus, a Professor of the University of California at Irvine, is the latest scientist to found his own company in hopes of treating or curing baldness. Plikus recently told the media organizationdot LAthat he hopes to put a topical solution on the market which would require less than daily applications to be useful. The name of Plikus’ startup company is Amplifica. The name has a nice ring to it, let’s hope that its clinical results will also be as pleasant.
The research which drove Plikus to this new venture involved studying hairy moles also known as hairy nevi. You may be familiar with the phenomenon yourself, moles which grow in isolated areas on the body and sprout several thick hairs or more. Plikus decided to investigate the biology of these hair moles to see if he could find which molecules were responsible for the hair growth. While his exact findings have not been publicized to date, it would seem that he is confident in his discoveries thus far. Veteran hair transplant surgeon Dr. William Rassman has joined Plikus as afounding memberof Amplifica. The company reportedly incorporated with a $500,000 investment.
$7.5 More Million Raised By Stemson Therapeutics & Patent Approved (9/16/20)
The United States’ current best bet to bring a hair cloning therapy to market this decade has attracted another multi-million dollar investment. This time, the investment comes from Allergan Aesthetics and UK based VC firm Fortunis Capital. According to today’spress release, Stemson will use the funding to conduct further preclinical work and expand its management team.
It was also noted that apatentcovering Stemson’s method of differentiating iPSCs into dermal papilla cells was recently granted which most likely spurred the completion of the latest funding round. Previously, Allergan invested $3M into Stemson back in June of 2019.
组织原完成第二次注射试验(9/10/20)
Today, Histogen announced that it has reached the 6 week treatment milestone among all participants in its ongoing clinical trial. This means all 36 male subjects have received their second set of injections and will receive one more injection set before completing the trial.
Histogen CEO Richard Pascoe also mentioned in today’spress releasethat the company is still on track to release topline data from the trial by the end of this year. The current phase 1b/2a trial of HSC is the most important trial to date for Histogen and could possibly lead to a strategic partnership if results are positive.
Angela Christiano 3D Printing Hair & Skin Interview (9/1/20)
Renowned researcher Dr. Christiano discusses her work in 3D printing human skin and hair follicles in this recent interview with the International Society of Hair Restoration Surgery.
Arena Pharmaceuticals Enters Phase 2 For Alopecia Areata (9/1/20)
The publicly traded Arena Pharmaceuticals announced today it has dosed the first patient in a phase 2 trial to evaluate the drug etrasimod in moderate to severe alopecia areata. Etrasimod is an oral sphingosine 1-phosphate receptor modulator. This is the first new approach for alopecia areata that we’ve heard of since JAK inhibitors become a hot topic several years ago. Full press releasehere.
WAY-316606 Cosmetic Trial To Be Published (8/31/20)
2022世界杯附加赛预测英格兰vs美国皇冠比分卵泡的思想已经从一个领导三英洁具tist at Giuliani Pharma that a peer-reviewed paper of a clinical trial involving a WAY-316606 analog will be published soon. The trial results will give us an indication of the efficacy of a topical WAY-316606-like cosmetic product that is planned to be released by the end of the year. Head to the main page to read more details on this development.
博士教授发现头发生长的化合物(8/30/20)
In a new Youtube video, our friend Youngjet from Japan shares more exclusive information about Dr. Tsuji’s hair growth research & developments. The latest update comes from Dr. Tsuji’s online lecture given on August 25th for the Adva Corporation of Japan. In the video, Youngjet describes 3 compounds which Dr. Tsuji has been testing for potential hair growth effects. One of the compounds outperformed minoxidil in a mouse study, though, we have learned in the past that mouse studies do not always translate well into humans. Youngjet mentions that Tsuji claims the new compound could become available “in 5 years”, this hints that the compound will be developed as a drug rather than a cosmetic if it is indeed commercialized. See the full video below and support Youngjet by subscribing to his channel.
CK Biotech To Commercialize PTD-DBM Treatment (8/21/20)
Head over to the main Articles page to check out the stickiedarticleon Dr. Kang-Yell Choi’s startup CK Biotech which is developing multiple therapies based on CXXC5-Dvl protein interference, including a treatment for hair regrowth. If successful, this new small molecule may be an ideal companion to dermarolling and microneedling to enhance new hair follicle formation.

MicroRNA-218-5p (8/20/20)
Before these past two weeks, it had been a while since I’ve received multiple emails and social media messages from my readers about a piece of hair regeneration news in the media. Something about the research from North Carolina State University seemed extra appealing to a lot of people. Insummary, a research team at NC State led by Dr. Ke Cheng compared the hair growth of effect of dermal papilla cells cultured in 2D, dermal papilla cells cultured in 3D, and minoxidil in a mouse model. Not surprisingly, the 3D cultured DP cells created the best hair regrowth response in the mice. Cheng and his team then went a step further to examine the exosomes secreted from the two sets of cultured DP cells. The 3D cultured DP cells were found to have an abundance of a specific microRNA called “miR-218-5p” which is likely responsible for those cells’ enhanced hair growth effect.
As you noticed, I’ve taken my time in sharing this news on Follicle Thought. The research is very interesting, but only time will tell if a single mRNA can become a truly effective hair growth therapy. A topical ointment involving miR-218-5p is feasible in the future, but it is a long ways off from this year. Cheng’s next step is to test miR-218-5p’s effect on hair growth as a solo ingredient. We’ll keep an eye on this as things progress.
Dr. Tsuji Hair Cloning Update (8/9/20)
Organ Technologies (with Dr. Tsuji) is one of the most interesting companies in the hair restoration field. For the past several years, Dr. Tsuji has been widely known for his work on growing hair follicles in the lab. After a long period of public silence, Dr. Tsuji made a presentation to Japanese graduate students and members of the public on August 5th. The lecture was attended by Youngjet, a Youtuber and hair transplant consultant. Head over to the main Articles page to hear what was shared in the presentation and excerpts from my personal conversation with Youngjet.
Kintor Completes US Phase 1B Trial For Pyrilutamide (8/4/20)
Yesterday, Kintor Pharmaceuticals completed an important US phase 1b trial of pyrilutamide (KX-826) in androgenic alopecia. The trial comprised 40 male subjects with 32 of the subjects receiving doses of pyrilutamide and 8 subjects receiving the control. Four dose levels of topical pyrilutamide were tested during the trial including 2.5 mg, 5 mg, 10 mg, and 20 mg, respectively. It will be interesting to see how the higher doses of pyrilutamide were tolerated by the trial participants.
In its latestpress release, Kintor says it is now analyzing the data from the trial and will be releasing a case study report in Q4 2020. As a reminder, this trial was designed to only evaluate the safety, tolerability, and pharmacokintetics (how the drug chemically affects the body) of KX-826, not efficacy. However, let’s not forget there is a phase 2 trial of pyrilutamide currently underway in China which will measure hair growth efficacy and results are due early next year if no delays have been incurred. Things are moving along for Kintor and their potential finasteride replacement, pyrilutamide. I’m looking forward to the Chinese phase 2 results.
Stemore Working On Cloning & More (7/28/20)
The South Korean company Stemore has some fantastic new research & development going on. Their pipeline includes multiple drug candidates, a dermal papilla cell therapy, an adipose stem cell therapy, and a 3D hair organoid cloning therapy. Head to the main Articles page for more details.
Histogen Trial All Set For 2020 Results (7/19/20)
The current phase 1b/2a trial of HST-001 taking place in Southern California has reached its goal of patient enrollment as of July 14. This is great news and means that the trial is on schedule to be completed by the end of the year. Histogenrecently announcedthis milestone on the investor relations section of their website. In total, there are 36 male patients who are participating in the trial over a period of 26 weeks.
As a reminder, this is the first time Histogen has included 3 separate sets of injections in a clinical trial which means that results could be better than ever before. We will have to wait and see, though. Topline data is expected in Q4 2020.
HairClone Receives Second Grant (7/16/20)
Earlier this month, HairClone announced it has received the “Covid Continuity” grant from InnovateUK, a British government agency. This is actually the second grant HairClone has been awarded by InnovateUK as they also received a grant back in January 2020. The funds will be used to support the company’s ongoing mission to set up an MHRA licensed facility to expand dermal papilla cells in. I had a brief conversation with HairClone CEO Paul Kemp regarding the latest grant and he shared this detail about the current projected timeline to begin services:“我认为延迟和持续的可能性d local lockdowns would mean that we are slipping into Q3 for the cell expansion service unfortunately, but we could possibly play catch up if things get back to normal more quickly than all the indicators are suggesting.”This is still a very reasonable and fortunate timeline, it’s much faster than the same therapy could achieve anywhere else in the world. Below is the tweet sent by HairClone.
Very happy to have been awarded a “Covid Continuity” grant from InnovateUK. This was very competitive and we were judged on
The quality of our ongoing project and satisfaction with progress as well as their judgement on our potential for the future. Great 3rd party validationpic.twitter.com/FqPbb3wdu3— HairClone (@HairClone)July 2, 2020
RiverTown RT1640 Published For Grey Hair Cure Research (7/13/20)
Check outthis articleon RiverTown Therapeutics which was recently updated to include information on a peer-reviewed study involving its candidate drug RT1640.
Triple Hair Corporate Video (7/3/20)
The intriguing newcomer company Triple Hair has recently produced an introductory video for their company. The video contains commentary from the executive team members of Triple Hair about the company’s background and future plans. I did not notice any new information from what was shared in my exclusive article a few months back. Nonetheless, It’s interesting to check out any content that a hair growth company puts out.
New Drug Screening Work At Koehler’s Lab (6/29/20)
Progress continues to flow from Dr. Karl Koehler’s laboratory in Boston. One of the important outcomes from the published study on hair-bearing skin this month was the opportunity for companies around the world to test out compounds for hair growth and hair loss prevention on actual human hair follicles instead of using outdated mouse models. We have seen in the past that positive results in mice do not always translate to humans. That research hurdle should now become a thing of the past as hair follicle in-a-dish testing is set to become the new gold standard. I recently contacted Dr. Koehler to see how quickly this work is coming along and am happy to relay the details I received.
The good news Dr. Koehler shared with me is:
- Multiple companies are in discussion with the Koehler Lab regarding testing new hair growth compounds on cultured human hair follicles.
- The point above means there are many potential hair growth drug candidates which we have never heard of before which could potentially enter the industry pipeline soon pending test results.
- Dr. Koehler and his team have several compounds of their own which they are preparing to screen soon.
We hope to hear more good news from the Koehler Lab in the coming months after some tests have been done.
Koehler’s Hair Cloning Breakthrough Published (6/21/20)
If you haven’t had a chance to read the latest article on Karl Koehler’s lab grown hair follicles made from all human cells, head on over to the main Articles page now. Koehler’s achievement has been widely recognized across the media over the past several weeks and was even evaluated by Dr. George Cotsarelis in a guest article for Nature. I was lucky enough to receive some exclusive information from Dr. Koehler regarding the next steps for this research and partnership interest from around the industry. This is work we will be following closely over the next several years as it is a game-changer in the making.
Inventage Labs And Withus Pharma Partner For Finasteride Injections (6/15/20)
本月早些时候,宣布我们相比Pharmaceutical Co. entered an agreement to manufacture Inventage Labs’ innovative microsphere-encapsulated finasteride injections. This new version of the drug will be used in clinical trials in South Korea. The finasteride polymer microspheres first made headlines in late 2019 after a peer-reviewed study showed favorable results, although in mice. Still, many people are looking forward to the development of this product which could limit administration of finasteride to once a month or every 3 monthsaccording toInventage Labs.
Applied Biology Minoxidil-Response Boosting Product Update (6/14/20)
Over the past several months many readers have asked for an update on Applied Biology’s minoxidil-adjuvant shampoo product which was planned to be released sometime in 2020. Initial interest in this product peaked late last year following an interview with Applied Biology’s founder Dr. Andy Goren which was published here on Follicle Thought. Over the past two weeks I have gotten back in touch with Dr. Goren who has been busy publishing new research onsulfotransferase enzymesand relations between androgen levels andCovid-19.
As a reminder, sulfotransferase enzymes are the enzymes located in the outer root sheath of hair follicles which help minoxidil to create a hair growth-promoting effect. While researching these enzymes I discovered a published study which showed that tretinoin is an upregulator of sulfotransferase activity, this led me to wonder if tretinoin was the ingredient in the upcoming AB product.
I made sure to ask about that topic in my recent exchanges with Dr. Goren, and thus, have this brief update to share as follows:
- The molecule in the upcoming product from Applied Biology is not tretinoin (and is assumedly more efficacious than tretinoin)
- Product is still planned to be released in 2020 according to Dr. Goren
- Likely to be a shampoo
Follicum Phase 2 Has Resumed From Coronavirus Pause (6/9/20)
The clinical trial news continues to roll in. Yesterday, Follicum announced via press release that the phase 2 trial for its hair growth peptide FOL-005 has recommenced after a long break due to coronavirus. To help make up for lost time, the company has enlisted a third study center in Germany to fill up recruitment as soon as possible. Check out the Follicum article currently stickied to the top of the main page to get details on the duration of the trial and when results are expected.
Follica设置阶段3 (6/5/20)
Follica of the PureTech Health Company announced its plans for a phase 3 trial in 2020, yesterday. Head over to the Articles page if you have not already read the trial design details for Follica’s FOL-004 hair regeneration treatment.
Histogen Doses First Patient With HSC In 2020 (6/2/20)
Yesterday, on June 1st, Histogen announced via press release that the first male patient has been dosed in their latest trial of HST-001 (HSC) for male pattern hair loss. Additionally, Histogen officially became a publicly traded company last week and is now trading under the name (HSTO) on Nasdaq. If Histogen can make such progress in 2020, then the sky is the limit for the rest of the industry.
Cassiopea Continues Toward Breezula Phase 3 Trial (5/28/20)
Cassiopea S.p.A. sent out a newsletter to announce its first quarter 2020 results, today. At the top of the newsletter, two bullet points addressed the company’s plans for its alopecia programs in men and women. Theexactstatements are as follows:
- “Filing of a Special Protocol Assessment with FDA for the phase III program for Clascoterone solution 7.5% in males
- Due to COVID-19, approximately three month interruption in the recruitment of subjects in phase II trial of Clascoterone solution in females”
Aspecial protocol assessmentis a request from a company to the FDA for approval of the design of a phase 3 clinical trial. This way, the company can be ensured that if successful their therapy will be eligible for regulatory approval following the trial. Also, it sounds as though enrollment for Cassiopea’s phase 2 trial for female alopecia has recommenced as enrollment began around December 2019, therefore a 3 month delay would have set them back to early April at the latest. Good news from Cassiopea as progress continues to roll in the hair restoration industry.
Kintor Pharmaceuticals Develops KX-826 (5/27/20)
The Chinese biotech Kintor Pharmaceuticals is developing two very interesting drugs which block out the signaling of androgen receptors. KX-826, one of the drugs in the Kintor pipeline, is aimed at treating hair loss. The company currently has trials going in US and China for both drugs and more plans in store. Head over to the Articles page to get all the details on Kintor’s candidate drugs and clinical trial schedule.
HST 001 / HSC Trial Now Recruiting (5/20/20)
Histogen’s upcoming trial of HSC in males is now recruiting. Please head to the main Articles page to get the contact and clinical trial site information.
Histogen Eyes Trial In June 2020 (5/19/20)
See the latest Histogenarticlefor all the latest details on one of the most unpredictable therapies in biotech history.
Fat Stem Cell Extract Making News For Hair Growth (5/18/20)
A new study using adipose-derived stem cells to treat hair loss / regrow hair was published in the Stem Cells Translational Medicine Journal today. So far, many news organizations in the UK and worldwide are covering the topic. Researchers of the study from Pusan National University Yangsan Hospital in South Korea prepared an adipose-derived stem cell constituent extract (ADSC-CE) which was applied topically twice daily by the trial participants. The ADSC-CE was basically prepared by culturing or multiplying adipose-derived stem cells, then disrupting their cell membrane using low frequency ultrasound, and then filtering out the membrane and debris. What’s left is the ADSC-CE which contains stem cell proteins.
The investigators of the studyreportpositive results as follows:
- 34 participants (mostly men) completed the study.
- At 16 weeks, the group who received the stem cell extract showed a 28.1% increase in hair count, compared to the placebo group who showed a 7.1% increase in hair count.
- The stem cell extract receiving group showed a 14.2% increase in hair diameter, compared to the placebo group which showed a 6.3% increase.
This image of 4 trial subjects, 2 male and 2 female, was included in the study PDF.
I would not say the above results are jaw dropping, but the statistical data does show that hair count and diameter did improve for the treated group. I notice a visible increase in hair density for the man on the lower left, even besides the fact his hair is longer you can notice more hair growth in the left corner of his crown from the 2nd to 3rd picture. Hopefully this work will lead to a new topical hair growth product derived from fat stem cells. The fact that this team cultured the ADSCs to make their product is impressive/innovative.
HairClone Shares Update (5/7/20)
Earlier this week, HairClone of the UK shared their first official update of 2020. Along with releasing a newsletter, the CEO of HairClone shared some exclusive commentary with Follicle Thought. It appears that the coronavirus situation has not delayed their efforts by much, so far. Head to the Articles page to hear what the latest date projection is for HairClone to begin providing cultured DP cells in the UK.
Thanks For Your Support (4/29/20)
I’d like to take a moment to thank some recent supporters of this site. Two long-time readers decided to give back to Follicle Thought this month and it’s much appreciated. Both opted for a one-time contribution. If you’re a fan of the content here and would like to support the mission of Follicle Thought please visitmy Patreon page, all contributions are appreciated.
Histogen Still Optimistic For Phase 2a Trial To Begin Soon (4/27/20)
At the end of last week I reported that Histogen has been active with their HSC program. The company has recently filed an amendment for their Investigational New Drug application for HSC and also announced that they are planning to initiate a new study for males sometime this summer. It would be quite ironic if Histogen is able to make progress during the coronavirus situation while many other companies are slowed. The recent Histogen article on the main page has more details and a link to the news release.
Canadian Startup Seeks Combination Therapy For Hair Growth (4/22/20)
In case you missed it, last week Follicle Thought debuted an exclusive look at the new company Triple Hair of Canada who is seeking to make some moves in the hair growth treatment industry. The company’s flagship therapy is called TH07 and combines 3 known molecules with hair growth properties. Besides that, Triple Hair also has 2 new candidate molecules in their pipeline and a patented all-natural formula which should be hitting the market in USA and Canada early next year. Head over to the Articles main page to get more details on Triple Hair’s offerings.
New TissUse Neopapillae Paper Published (4/16/20)
Today, a team of researchers from the German company TissUse published a new scientific study on the company’s hair cloning technology. Thestudy appearedin the Journal of Tissue Engineering and Regenerative Medicine and is titled “Reconstructed human skin shows epidermal imagination towards integrated Neopapillae indicating early hair follicle formation in vitro.”It is fantastic news to see the TissUse team publishing new hair cloning research in 2020. Renowned hair research scientist Dr. Kevin McElwee commented on the new study “So far not full follicle formation, but they are making good progress. Some additional differentiation factors need to be added to promote full follicle formation.”
Like I’ve said in the past, whenever a study is published in a scientific journal the researchers have progressed past what was published. Great news from TissUse!
Phase 3 Trial For Alopecia Areata Planned (4/8/20)
Concert Pharmaceuticals, a biotech company focused on the repurposing of approved drugs, recently announced plans to initiate two phase 3 trials for its alopecia areata program. The trials are expected to commence in Q4 2020 pending resolution of Covid-19, according to the company. CTP-543, an oral selective inhibitor of JAK 1/2, is Concert’s drug being evaluated. Importantdetailsabout the upcoming trial are as follows:
- Eligible participants will be ages 18-65 with ≥ 50% hair loss
- Approximately 700 patients expected to enroll in each of the two Phase 3 trials
- Dosing will include 8 mg twice-daily or 12 mg twice-daily of CTP-543 or placebo for 24 weeks
- Primary endpoint: Percent of patients achieving a SALT score ≤20 at Week 24
To read more about Concert’s alopecia areata drug development program or inquire about becoming a trial participant please visit Concert Pharma’swebsite.
L’Oreal Makes Progress On Hair Cloning (3/27/20)
Earlier this week, Follicle Thought presented an exclusive poster presentation from L’Oreal titled “A combination of hair matrix cells and dermal papilla spheroids self organizes to form hair follicle organoid in vitro.”This work is indeed an effort at creating hair follicles outside the body, commonly referred to as “hair cloning.” The poster is likely the first public update L’Oreal has made on the subject of hair cloning since announcing their collaboration with the Poietis company in 2016. Head over to the main Articles page to see the full poster which involves no mice and read more details about L’Oreal’s efforts to become a leader in the hair follicle cloning industry.
Replicel Posts Commentary On Shiseido RCH-01 Trial (3/23/20)
Today, Replicel issued a press release in reaction to Shiseido’s publication of its RCH-01 trial results in the JAAD journal on March 5th. In the release, Replicel maintains a positive attitude towards the results which were shown and points out possibilities for improving the treatment’s administration in the future. Replicel also states they are committed to using their dermal injector device (RCI-01) for future hair growth and skin rejuvenation trials, but do not mention any specific plans to conduct trials in the near future. It will be interesting to see if RCH-01 or other hair growth injectables will be tested using the RCI-01 device someday. The full release is available on Replicel’swebsite.
New HairCell Results (3/20/20)
The HairCell website was recently updated with new results from their trial involving bioelectric stimulation and PRF injections in South Africa. I’ve updated the original HairCell Pilot Trial article to include new images and also link to the HairCell web page which contains all of the new trial results. I found trial subject “Jason” to show a meaningful improvement of hair density, particularly at his crown after undergoing the HairCell procedure. There are now plumes of hair forming a spiral pattern in his crown where it was mostly bare before treatment. Head over to the Articles main page to check out the new data from HairCell.
Follicum Topical Phase 2 Trial Now In Progress (3/12/20)
The first few members of Follicum’s latest phase 2 trial of FOL-005 have begun dosing, according to the Swedish biotech company. The announcement was made this week via press release. All of Follicum’s previous efforts have lead up to this important trial, where the FOL-005 peptide will be tested topically on human scalps. The outcome of this trial will be important for the industry, as we will find out if a new viable option to treat hair loss with will be available in the next few years. Head to the main Articles page to get full trial details and a timeline for its results to be shared. (Note: This trial is temporarily suspended due to Coronavirus / Covid-19. The trial is likely to recommence within 2 months.)
Shiseido To Conduct More Clinical Trials For RCH-01? (3/9/20)
Over the past weekend, the revelation of Shiseido’s long awaited trial results hit the internet. The trials involved single-injection doses of multiplied dermal sheath cup cells and many readers across the internet were not overly impressed with the results that they obtained. This would make the second trial of RCH-01 which failed to create a “wow” factor and many might have guessed it would be the last, however, Shiseido seems to say otherwise. A Japanese press release was discovered by Fuji Kagurazaka of HLC100 and the release states some interesting facts about Shiseido’s future plans for the treatment. Head to the latestShiseido RCH-01 Trial Resultsarticle to get the full details.
Bacteria Research Could Have Potential As Hair Loss Treatment (3/5/20)
Researchers in Taiwan have identified a novel method of reconverting estrogen hormones into androgen hormones via a bacteria strain from theDenitratisomasp. genus known as DHT3. It’s interesting that this particular type of bacteria contains the letters “DHT” in its name. The asbstract of thepublished study“Retroconversion of estrogens into androgens by bacteria via a cobalamin-mediated methylation” states that DHT3 is “capable of catabolizing estrogens or androgens anaerobically.” To catabolize simply means to break down a substance into simpler substances. In a media article published by theTaipei Times, lead researcher Dr. Chiang Yin-ru states “Specific hormones regulate the development and maintenance of male and female characteristics, and the team’s research signals potential new treatments for menopause, baldness and prostate cancers through probiotics.”The article also mentions that Dr. Chiang and his team are already working on the development of such probiotics.
This research may be a bit of a long-shot as a successful treatment for male and female pattern baldness, however, no method should be disregarded in the search for new effective treatments. Hopefully we hear an update about Chiang and his team successfully developing a new probiotic this year.
Samumed Completes Enrollment For Phase 3 Trial (2/28/20)
在公司简报发表在2月28日2020, Samumed announced that their phase 3 trial of SM04454 for AGA has finally reached its quota of trial participants. That quota was a whopping 625 male subjects between the ages of 18 – 45 years old. It’s no wonder that it took Samumed roughly 15 months to fill up enrollment for this pivotaltrialin Turkey. The last few subjects who enrolled in the trial will still have to complete about 48 weeks of SM04554 application which will extend the trial into January 2021. We can certainly expect results from the trial to be shared at some point in 2021, which is good news.

Investors For Breakthrough Hair Growth Therapies (2/27/20)
The demand for a new effective hair growth treatment is at an all-time high in 2020. As is the case in virtually all research fields, the best hair growth technologies are discovered by smaller startups and research labs. Follicle Thought is in contact with several of these groups which have yet to announce their efforts in creating next-generation hair growth therapies. In order to move their projects into worldwide successes these researchers are in need of investment capital. If you happen to be an accredited investor who is interesting in hearing more about these timely opportunities please get in touch through the世界杯2022入围名单 page.
Media Article On European Hair Loss Cure Companies (2/19/20)
今天发表的一篇文章里,2月19日2020, on several European based companies who are developing treatments for hair loss or baldness. The companies featured in the article include Cassiopea, Follicum, and Giuliani. Most of the information covered in the article will be familiar to the audience of Follicle Thought, however, there were a few interesting points made and details worth noting below.
- Both Follicum’s and Cassiopea’s treatments may become suitable options for female pattern hair loss.
- Follicum’s FOL-005 is a peptide based treatment which does not interact with the hormone system. Thus, if it works well for men it should also work well in women with no risk of altering gender-specific hormone profiles.
- Cassiopea’s drug Breezula blocks DHT’s interaction with hair follicles locally rather than systemically like finasteride does, thus, it presents a safer, more practical approach for female patients. The company has even initiated a phase 2 trial specifically for women which is testing Breezula against the status quo 2% minoxidil solution.
- Ralf Paus noted that his team has identified 2 or 3 compounds which work on the Wnt pathway to promote human hair growth in culture. Could it be that originally there were more drugs than just WAY-316606 identified to mimic the hair growth effects of cyclosporine A? Paus mentioned that toxicity studies and then a clinical trial were the next steps for his candidate compounds.
Original article foundhere.
Follicum重要获得监管部门的批准Trial (2/3/20)
我们的朋友在Follicum收到批准摇来摇去m the German medical agency BfArm (equivalent to FDA) and the German Ethics Committee to begin an important phase 2a trial for its FOL-005 drug. This trial is particularly important for Follicum because it is the first time their peptide FOL-005 will be trialed topically for hair growth. In previous trials, the peptide was injected into human thighs and scalps, both with positive results on hair growth.
According to Follicum’s latestpress release, the trial will include 200 patients who will receive treatment of FOL-005 or placebo for a period of four months. Results from the trial are expected in Q4 2020. While the trial has not officially started yet, we can expect patient recruitment to fill up quickly and the start of the trial to commence within a week or two. There will be a full article published when the trial begins this month.
Histogen Merges With Publicly Traded Company (1/31/20)
On January 28, 2020 Histogen announced it will be completing a full merger with Conatus Pharmaceuticals. For a complete recap of the deal and its implications, please visit the main Articles page on Follicle Thought. One of the major outcomes of the deal is Histogen will soon be a publicly traded company on Nasdaq. This means members of the public could now buy stock in Histogen if they chose to do so. Publicly traded stock could potentially solve Histogen’s financial woes which have been prevalent over the years for the company. This latest press release also mentions a time period for Histogen to initiate an upcoming phase 1b/2a trial for HSC in male pattern baldness.
大学研究RiverTown新闻(1/29/20)
More grey hair cure research is in the news and this time it involves a compound which is also intended to regrow hair. That compound is none other than RT1640 which has been featured in multiple articles on Follicle Thought for its abilities torestore pigmentto hair which has gone grey and toregrow hairin balding areas. Over the past two years, RiverTown has been involved in a collaborative research project with the lab of Dr. Melissa Harris of the University of Alabama. The collaborative project is intended to study RT1640’s ability to restore pigment to hair follicles which have turned white or grey due to loss of melanocyte stem cells. This week, Dr. Harris appeared on a morning news broadcast to discuss her latest work with RiverTown as seen below. The link to the interview can be found in the latest RiverTown article on the main page of Follicle Thought.

Grey Hair Cure Research Update (1/23/20)
Most of us are familiar with the sentiment that stress can cause grey hair; you’ve likely heard this since childhood from your mother or relatives. What’s interesting is that this widespread belief was not scientifically understood until recently. On January 22, 2020, Scientists from the Universities of Sao Paulo and Harvard published their findings on how stress leads to a depletion of the stem cells which give hair its pigment.
The scientific article is titled“Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells”and was published in the Nature journal.
In the study, Hsu et al. injected mice with a naturally occuring chemical called resiniferatoxin which is derived from a cactuc-like plant. Injections of resiniferatoxin provided a reliable and uniform way of boosting stress hormone levels across the group of mice. After receiving injections of the compound the mice began to display premature hair graying, and even mice who lacked immune cells or adrenal glands displayed the hair graying effect. This showed that the immune system was not the major cause of the hair graying process which lead the researchers toinvestigatethe sympathetic nervous system’s role in the loss of hair pigment. It turns out that norepinephrine, which is produced by the sympathetic nervous system during stress, causes an overstimulation of melanocyte stem cells. This causes the melanocyte stem cells (cells which give hair its color) to produce more pigment than usual and leads to their permanent depletion. While a new treatment to prevent this biological response is not imminent, it does provide scientists another target to aim for in the quest to keep people looking younger and feeling better.
J. Hewitt Hair Multiplication Trial Update January 2020 (1/21/20)
Many readers have been asking for an update on J. Hewitt’s trial of the Smart Hair Transplant (SHT) procedure which was planned to begin in December 2019. The proposed start date of December 2019 was given by J. Hewitt’s CEO Jon Knight who shared this information with Follicle Thought, Fuji Kagurazaka (admin of Hair Loss Cure Japanblog) and other hair journalists. Unfortunately, the trial was not able to begin in December and is now facing some delays.
I have recently been in contact with CEO Jon Knight to get clarity on the cause of the delay and current status of the trial. In short, J. Hewitt’s trial was not able to begin as planned because negotiations with a Cell Processing Center (company who would conduct the trial) fell through after long negotiations. This now accounts for about 6 months of lost time. Jon Knight provided this brief quote regarding the trial for readers: “We have encountered some unexpected delays in the process of establishing an agreement with a Cell Processing Center. Our goals remains the same and J. Hewitt continues to pursue its trial for SHT in Japan.”
While it is disappointing to know the trial was not able to begin as planned, it is good to know that J. Hewitt is still actively working to initiate the trial. The time range for the start of the trial is unclear for now and it’s most likely best for the company to not give another estimated date just yet. So, perhaps the initial announcement by Knight was a bit overconfident, but it is clear that J. Hewitt hasofficially partnered with TissUsefor the sole purpose of trialing the SHT technology in advantageous Japan. As an aside, J. Hewitt is not a publicly traded company and would gain no financial benefit from their previous announcement regarding clinical trials. I will provide another update about the SHT trial as soon as new information becomes available.
Ultimate Hair Treatment Guide Updated 2020 (1/13/20)
The industry’s flagship hair loss cure article has been updated for 2020. Most of you know it as the “Ultimate Guide to Hair Regeneration.” For the first time in about 5 years Replicel is no longer at the #1 spot of the list. Luckily, a new front runner for the guide was not hard to find. Another noteworthy change was the removal of Aclaris Therapeutics from the listings. For those unaware, Aclaris has indefinitely suspended their programs for alopecia pending the success of finding a larger clinical partner for the programs. Some good news for the guide is the addition of the Stemore company from Korea who is planning to enter a clinical for an innovative dermal papilla therapy later this year.
The Ultimate Guide pipeline chart was also updated as seen below.

HairClone Receives Funding (1/8/20)
Welcome to the first update of 2020. Yesterday, HairClone announced that they have received a special grant from the UK government to carry out their work in cloning hair stem cells. This is a great comeback story as HairClone had failed at a crowdfunding campaign in 2019. The grant money is said to cover the expenses that the crowdfunding was intended for. HairClone is also now working with a British University on the project. Head over the Articles page to read the whole story and get the latest timeline for HairClone injections development.
Video Update From NGF User (12/31/19)
Today, in the comments section of the latest ‘2020 Look Ahead’ article, a reader shared a link to a Youtube video posted by a user of the NGF-574H Medipost/Celino product. The person who uploaded the video has been sharing updates of his experience with NGF-574H over the last 5 months. His previous videos did not show much contrast in his before and after photos, but, in the latest video from December 30th, there is noticeable improvement in his crown area. The user notes that he has began dermarolling (microneedling)in the past month and believes that it has improved the efficacy of NGF for him. I’d like to hear what the audience thinks about this video.
Click hereto see the video from the NGF-574H user. Feel free to share feedback about the video in the comments of this thread or the latest 2020 Look Ahead article where the discussion started.
Dermal Sheath Muscle Research (12/26/19)
New research has been published by Dr. Michael Rendl and his team at the Icahn School of Medicine at Mount Sinai Hospital. Dr. Rendl is a renowned researcher in hair growth industry and currently serves as on the Advisory Board of Stemson Therapeutics. His latest finding points to another mechanism that could be exploited to keep hair follicles growing and prevent hair loss.
For the first time ever, Rendl and his team have identified thedermal sheath as a smooth musclewhose contractions lead to the shedding of hair shafts from the follicle. The interesting point here is Rendl believes that certain drugs could block the contraction of the dermal sheath muscle which would prevent hair shedding and keep follicles in the anagen (growth) phase. Rendl also thinks that this latest discovery could inspire a new focus in the hair research field.
Rendl’s published study titled “Dermal sheath contraction powers stem cell niche relocation during hair cycle regression”was published this month in theScience magazine.
2020 Hair Growth Treatments Look Ahead (12/23/19)
Last week I published the annual preview article covering projected treatment developments in 2020. Some of the most anticipated developments are a hair multiplication trial which is scheduled for the 1st quarter of 2020 and a cosmetic WAY-316606 topical has already begun human testing. With so many hair cloning companies at the brink of human trials we hope that 2020 will finally produce a long awaited breakthrough. The Articles page has the complete list of expected milestones in 2020.
Follica Announces Positive Data From Regimen Optimization Study (12/19/19)
Just before the closing out of 2019, Follica has announced positive data from its latest study of their FOL-004 treatment platform. This news comes shortly after the first set of before and after images of the FOL-004 treatment were shared and discussed around the internet. Today’s press release from Follica contains statistical figures of terminal hair count improvement from the treatment as well as a projected timeline for the start of its next phase 3/pivotal trial. Head to the main page for all of the info or head directly to the articlehere.
Support Follicle Thought On Patreon (12/9/19)
As we head into another Holiday season I would like to remind the readers and supporters of Follicle Thought of my Patreon donation page. MyPatreon pageallows readers to make a monthly financial contribution to this site in exchange for all of the work that goes into it. There is also an option to make aone-time gift donationto Follicle Thought which I have written a short how-to post about. All contributions, big or small, are greatly appreciated and support the mission of this website to play a part in helping new effective treatments receive the attention and resources they need to become a reality for us all. Thank you very much for choosing to support Follicle Thought in this way and Happy Holidays!
Stemore Joins The Race (12/5/19)
Yesterday, Follicle Thought debuted some exclusive information from the company Stemore of South Korea. Stemore (also spelled Stemmore) is developing novel cellular therapies for hair growth including dermal papilla cell, and cultured adipose derived stem cell therapies. The company has even created an edited adipose derived stem cell which behaves more like a dermal papilla cell. On top of the cell therapies in Stemore’s pipeline, the company is also pursuing some over the counter hair growth products including a 5a-reductase inhibitor. Head over to the main Articles page to hear why the company got started when these treatments are going to enter clinical trials.
Replicel Year End Update 2019 (11/26/19)
Today, Replicel sent out its annual year-end review newsletter to shareholders and the public at large. When Replicel newsletters are published, most people are scouring them for one thing – a Shiseido update. Today’s press release does contain some information on Shiseido, however there is no date given for a release of Shiseido’s trial data and the companies remain with unsettled differences.
Here is a quoted section from Replicel’syear-end reviewnewsletter:
“Partnership with Shiseido – The dispute regarding the status of the agreement between Shiseido and RepliCel, for the license of RCH-01 in Asia, remains unresolved but is not the subject of any litigation or arbitration. RepliCel maintains the Agreement remains intact and has recently demanded that Shiseido share the data from the recently completed study of RCH-01 in Japan. With the completion of the Japanese RCH-01 study earlier this year, RepliCel is now actively engaging Shiseido to deliver the study data and reach a resolution to the dispute between the parties. RepliCel is eager to end the ongoing dispute and work with Shiseido to commercialize RCH-01.”
It’s good to hear that there is no litigation going on with the companies, but perhaps it may be time for some arbitration. Replicel mentions they are actively engaging Shiseido to things forward and that is good news. We hope to hear about a resolution and data release for RCH-01 as soon as possible.
Follica Before And After Photos In 2019 (11/22/19)
One of the most tight-lipped companies in the hair growth industry, Follica, has finally revealed a result from their ongoing optimization study of lead candidate FOL-004. The photos that were released in a company PDF presentation show a male subject at baseline and at 85 days post treatment (just under 3 months). Overall, the photos are receiving a positive response across the internet. When reading the article on the main page of Follicle Thought be sure to check out the link to a zoomed-in version of the photos for better comparison.The PDF presentation also gives more insight about the in-clinic portion of the FOL-004 treatment. Follica’s FOL-004 is moving towards a leading spot among therapies in development and should reach the start of a FDA-approving trial early next year.
$25 Million Investment Into Next-Gen Hair Loss Cure (11/17/19)
If one thing has been proven over the past 5 years in the hair growth industry, it is that Allergan will not be outdone. The company has now expanded its pipeline of hair growth therapy candidates which already included Stemson Therapeutics and Pelage Pharmaceuticals. In a record-setting deal, Allergan has now teamed up with Exicure to develop two innovative treatments for alopecia. One of the projects has been confirmed to be aimed at androgenic alopecia or common hair loss, and the other alopecia-related project is yet to be announced. Obviously, alopecia areata or chemotherapy-induced alopecia are likely targets. Head over to the main page to read more about Exicure’s SNA based technology and view an informative video on the company.
Cassiopea Pursues Breezula For Women (11/14/19)
The anti-androgen drug which showed positive results in a phase 2 trial for men earlier this year is now going to be clinically tested in women. For full trial details and update on Cassiopea’s male alopecia program head to the Articles page.
Product Lineup From Applied Biology (11/5/19)
I recently had the pleasure of speaking with Dr. Andy Goren, President and CMO of Applied Biology. Dr. Goren is a world-class hair researcher and works on academia-based projects with leading universities around the globe. His company Applied Biology acts as a bridge for his academia research to extend into the world of commercialization. Our discussion was based on the current lineup of products Applied Biology’s pipeline and I’ve shared his sentiments about them in a recent article. This article has been particularly well received by the readers and this is perhaps due to an intriguing technology which is scheduled to be released next year. Dr. Goren has done extensive research on minoxidil response and sulfotransferase enzymes. Through this work Dr. Goren and his team have identified a molecule and several analogs which can improve a person’s hair growth response to minoxidil.
Read more about the product that his company is releasing to improve minoxidil response and several others in the latest article on the main page. Enjoy.
ISHRS World Congress 2019 (10/30/19)
The new program for the latest ISHRS 27th Annual World Congress has been shared online. I’ve taken a look through it and highlighted the most exciting research which is being shared at this years Congress. Topics of interest include allogeneic hair transplants, stem cell treatments for hair loss, cultured human hair follicles, and neopapilla. Head over to the main Articles page to read the full scoop on these presentations.
Taking A Look At Regenera Activa (10/28/19)
A new treatment using autologous stem cells is becoming popular across European countries. I’ve had several readers ask me about the treatment recently, and, coincidentally, the procedure has come up in conversations I’ve had with a couple of well known hair doctors as well. Because of this I decided it would be a good time to take a look at some of the results photos which are available for the Regenera procedure. On the latest article you can view the best available results images I was able to find. The Regenera Activa procedure has shown show interesting results for patients but for now it’s still too early to consider it as a viable option across the industry. Below is one of the best comparisons I was able to find, to review other results from this treatment head over to the Articles page.

Histogen Lives (10/25/19)
The hair growth company with tenure is pursuing a new trial for males with AGA. This week, Histogen made an investor related presentation in San Francisco,CA with hopes to raise funds to further three of their programs into clinical trials. The most important of these programs is the HSC product which most of us are well familiar with.To date, it’s been about 7 years since a male patient has been dosed with HSC and some wondered if it would ever make a resurgence. This could change soon as the company plans to begin a new male trial in the first quarter of 2020. Head over to the main page for the full article and to read how a new strategy by Histogen could finally get their HSC product into the clinic uninterrupted.
HairClone In News Again (10/22/19)
Shortly after Follicle Thought ran an exclusive HairClone article last week featuring commentary from the company’s CEO Paul Kemp, the company has popped up in the British news again. The Daily Mail newspaper published an article today on HairClone and their banking system. For the first time, an estimated price for injections was given. The article states that injections could cost around £3,000 to £7,000 each time given. I think the lower number would be pretty agreeable for most people in the DP cell injections could retain and rebuild follicles as intended. To read the full articleclick here.
New Injectable Form Of Finasteride Being Trialed (10/19/19)
A Korean biotech company has created yet another way of administering one of the world’s most widely used hair-loss drugs,finasteride.In recent years we have seen the trend of topical finasteride becoming more popular. Now, Inventage Labs is seeking to take local administration of the drug a step further through injections. To do this Inventage Labs is employing its proprietary drug delivery system called ‘IVL-PPFM’. The IVL-PPFM technology uses polymer-based microspheres to deliver long lasting therapeutic doses. In this case, microspheres containing finasteride are injected into scalp, and as the microspheres slowly degrade over time, finasteride is released into the surrounding tissue at a controlled rate. Thus, one injection of microsphere finasteride can last much longer systemically than an oral tablet.
Researchers at Chung-Ang University Hospital, led by Professor Kim Beom-joon, compared the effects of the new injectable finasteride to the standard oral version in two groups of mice over a 10-week period. The group who received one injection of finasteride showed a higher hair growth rate of 93.3% compared to 86.7% in the oral group. Also, blood DHT levels were similar between the two groups at the end of 10 weeks with just one injection.
Inventage Labssays human patients will only need one injection of finasteride per month to receive hair growth benefits and is currently planning clinical trials to confirm this. The company also thinks that there is less chance of side effects using injectable finasteride because much less intake of the drug is required compared to daily oral doses. The full scientific study was published in theInternational Journal of Molecular Medicine.
A Closer Look At HairClone (10/16/19)
Yesterday, I published a new article discussing the treatment approach of HairClone. In the article, I received commentary from CEO Paul Kemp on what led him to start the company and the scientific evidence to support its therapy. I felt this would be refreshing due to a seeming over-emphasis on the company’s hair follicle banking model in most online discussions. The hair banking is a creative system, and will be very useful for patients if all goes as planned, but, it should be coupled with the therapy and not overlook it.As we are now in October 2019 the need for newer effective treatments is as real as ever and HairClone represents a unique opportunity to get a cell based treatment into the clinic fastandshow results fast. Head on over to the main page to read the details on HairClone’s DP cell injections.
Stemson Therapeutics Featured In News Video (10/11/19)
One of the popular companies in the race to create unlimited hair follicles, Stemson Therapeutics, has been appearing in the media quite a bit recently. Last week, an affiliate of ABC news in Tampa Bay, Florida, ran a story on the subject of hair restoration. The segment featured a male hair stylist who is afflicted by hair loss and presented some potential options for him to restore his locks. One of those options was Stemson’s hair multiplication treatment, which we know is still some years away from clinical practice. Also, in the video below there is some footage of Stemson’s laboratory space and a brief appearance by lead scientist Alexey Terskikh PhD.
Yuva Biosciences Emerges (10/9/19)
A new spin-out company from the University of Alabama at Birmingham is working on treatments to thwart the symptoms of aging. One of their main targets for treatment: hair loss. Besides updates on late stage clinical trials, new companies adding to the mix of potential future treatments is some of the best news we can receive. Besides covering Yuva’s background and website informationI was also able to conduct a brief interview with the founder of the company, Keshav Singh PhD. During our Q&A, Dr. Singh discusses the regulatory path he is pursuing for Yuva’s hair treatments and the current status of product development. Head over to the Articles page to read more about this company’s plans for the hair industry.
Follicum Plans Next Clinical Trial (10/2/19)
One of the three main topical drugs in development currently, FOL-005 from Follicum, is finally scheduled for its next clinical trial. Following FOL-005’s previous trial which was administered by injections, the company has spent time developing an effective topical formulation of the FOL-005 peptide. Previously, FOL-005 showed the ability to increase human hair count and trigger the anagen phase in hair follicles. Follicum is hoping for even better results from a topical formula which can be applied conveniently on a daily basis. Head to the main page to find out when the next trial for FOL-005 will be taking place.
WAY-316606 Product In The Works (9/30/19)
One of the biggest news announcements of the year came this past week as we learned that Giuliani Pharma is now actively developing a product based on the WAY-316606 research. The product will contain a molecule nearly identical to WAY-316606, but will meet cosmetic regulatory guidelines. This means, if successful, we get the same mechanism of action, but about 5 years sooner than the drug version. It’s a smart move by Giuliani and could benefit us allAs far as upcoming products in the pipeline whether clinical or OTC, this product has to beone of the top candidates at the moment.
WAY-316606 was found to activate the Wnt pathway, one of the most important and researched drivers of the hair growth cycle. It’s easily the most scientifically backed candidate that was been discussed in the past 3 years. Head on over to the main page to get full details on human trials!
HairCell Initial Study Results (9/23/19)
HairCell是宣布一些你们公司ars back with a unique approach to hair growth. It seeks to use bioelectric stimulation to trigger the release of growth factors which are known to support hair growth. HairCell is looking to find out if it can trigger these growth factors effectively enough to create statistical improvement in hair density. So far, two male result photos have been shared and are featured in a new article on the main page. HairCell is preparing for an additional study and may alter the signals which are being used in the treatment.
Electrical Stimulation Cap May Help Hair Growth (9/21/19)
从威斯康辛大学的一位科学家,Xudong Wang, has created an electrical stimulation device which has shown the ability to stimulate hair growth in preclinical trials. Initial studies of the technology were published in theACS Nano journaland showed that the device triggered the release of keratinocyte growth factor and vascular endothelial growth factor, both known to promote hair growth. The device even outperformed minoxidil in the studies by growing longer and denser hair, although the test subjects for these results were mice. Perhaps more interestingly, Wang gave one of the prototype baseball caps containing the stimulation device to his father who has been losing his hair for several years. According to Wang, his father grew new hairafter one monthof wearing the cap. An interesting detail of the cap – it uses body movements to create electricity so there would be no need for a battery. Wang is currently preparing for a clinical trial in humans which would have a quicker regulatory path to market similar to “FDA cleared” laser cap devices. If this new device could produce results superior to laser caps, it would be worth a worthwhile addition to any hair growth regimen.
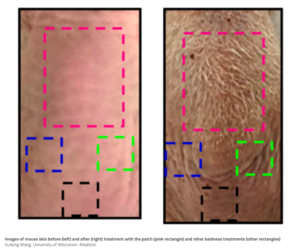
World Class Researcher Discusses Future Treatments (9/19/19)
One of the most experienced and knowledgeable hair loss researchers in the industry Dr. Ralf Paus, has recently shared some insightful commentary with Follicle Thought and its readers. In the article, Dr. Paus discusses some of his latest work which is expanding upon the sandalore discovery from last year, the WAY-316606 discovery, and a few other potential targets for hair growth. He also offers his viewpoint on the current next-generation therapies being developed for hair loss. The response on multiplied follicles may be a bit controversial to some, however it’s just a point of view for now. Paus was also featured in a recent Guardian article where he advocated for more funding in the hair loss research industry. If increased investments were to manifest in hair research, I think a lot of people would agree Dr. Paus’ lab should be one of the first destinations to receive support.
New Research For Chemo-Induced Alopecia Treatment (9/12/19)
Researchers from the laboratory of Dr. Ralf Paus at The University of Manchester have identified a new model for preventing chemotherapy induced hair loss. Taxanes are commonly prescribed cancer drugs which are widely known to cause hair loss in patients, sometimes permanent. Ina study published today, headed by lead author Dr. Talveen Purba, a new method of mitigating the damaging effects of taxanes on hair follicles has been identified. The scientists found that a class of drugs known as CDK4/6 inhibitors, also used to treat cancer, prevent cell division in the hair follicle which limits the ability for taxanes to damage the important hair follicle stem cells located in the outer root sheath. Thisarticledescribes the researchers “bathing organ-cultured hair scalp hair follicles in CDK4/6 inhibitors.” It’s curious to know if the laboratory has developed its own hair follicle culture model and what other plans it might have for that development.
Another important question that arises from this science: could it be applied to AGA to regrow hair as well? I hope to hear back from the author of this study soon to shed more insight.
Clarity On Samumed Trial (9/12/19)
Earlier this week, I shared a article discussing Samumed’s phase 3 clinical trial for its AGA drug SM04554. The trial is one of the more popular topics to watch in the field currently, due to the fact that phase 3 trials are hard to come by in the alopecia field. The latest news on Samumed’s trial came with a twist, as of now it seems that this trial would allow approval solely in Turkey and not the US which has been assumed until now. It’s possible that a further trial would be necessary in the US before the drug could be approved there. Thankfully we had such great news last week with J. Hewitt to tide us over. Even still, positive results in the Samumed phase 3 trial would most likely be enough to quell the concern over immediate geographic release of the product. Initial subjects of the trial will be completing treatment around mid 2020.
J.Hewitt To Trial Smart Hair Transplant In 2019 (9/6/19)
Earlier this week I launched news that J. Hewitt of Japan is planning to begin a groundbreaking clinical trial for the Smart Hair Transplant technology (SHT) by the end of this year. The SHT method was developed by University of Berlin scientists and then spun out into the company TIssUse. The team’s research is one of the most longstanding and respected models in the industry and first became popular after a scientific article was published back in 2011. The recent news of a planned trial in 2019 has caused a lot of excitement – rightfully so. If the trial comes to fruition it would launch J.Hewitt into the leading position among companies racing towards regulatory approval.
This week’s article has also prompted a lot of questions from readers who wish to know more specifics about the SHT technique and its plan of action. I’m preparing a few questions, listed below, that I hope J. Hewitt will be willing to respond to by the end of next week.
Questions include:
–Is the SHT designed to grow new hairs in bald areas or thicken existinghair?
– If new hair is grown, would there be need for a growth guide with SHT?
– Would a 5 person trial be sufficient to get this treatment approved in Japan or are further trials necessary?
– Do you have an estimated cost for this treatment at this time?
New Anti-Androgen Drug In The Works (8/29/19)
A pharmaceutical company with subsidiaries in both the US and China has emerged with a new topical drug candidate to treat pattern baldness. The aforementioned company is none other than Kintor Pharmaceuticals and their potential compound to treat hair loss is called KX-826. The good news is KX-826 has already initiated the clinical trial process which is a big plus. An article was published earlier this week containing specifics of the drugs mechanism of action and current clinical trial status. Head over to the main page to check it out or review the information. Within a few years time this drug has the potential to be a considerable development for treatment options.
RiverTown Alternate Angle Photo (8/24/19)
I wanted to share a little bonus angle photo of the familiar RiverTown Therapeutics trial subject to help get through the weekend. This photo was taken a few weeks after his previous 6 month photo. If you’d like to discuss this particular photo please comment on a separate page created for the photofound here.
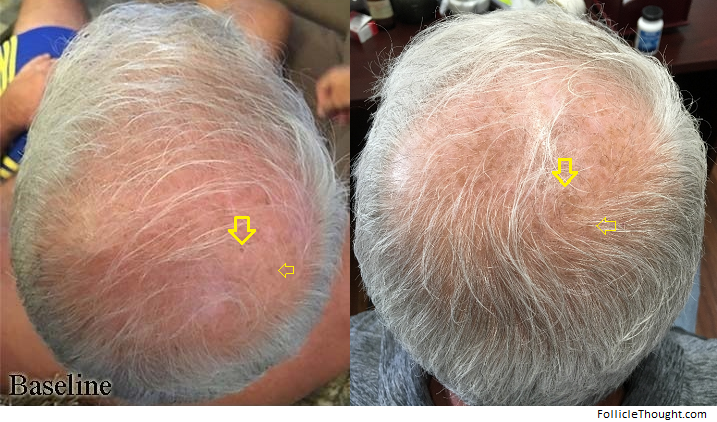
Japanese Blogger Shares English Tsuji Video (8/15/19)
The Japanese hair restoration blogger “Youngjet” has just added an English version of his popular “Tsuji Cure” video. In the video Youngjet proclaims that the Tsuji treatment will reach the market possibly in 2020-2021 and have a price tag around $300,000.
这刺激了很多网上的谈话,yes one question that has been posed by the readers is, are we jumping the gun here? Is the idea of a price before a clinical trial even relevant?Well, the price is certainly interesting to many people, it gives us an idea of how much this treatment may cost if and when it is approved. For a more concrete schedule we will have to wait and see when Organ Technologies releases their next press release to hopefully include a date for clinical trials.
Aclaris Seeks Partner To Further Program (8/14/19)
The latest press release from Aclaris Therapeutics states that the company is now in a position to seek a strategic partner for their alopecia program involving JAK inhibitors. The company had gained positive attention in June when it shared positive data from its androgenic alopecia trial, however several factors since then have caused the company to re-strategize its spending and therapeutic programs. More details and quotes can be found on the main page article.
HairClone Responses From Paul Kemp (8/9/19)
The latest news of HairClone starting up its follicle banking business has stimulated discussion about the prospective company again. This has prompted HairClone CEO Paul Kemp to join the discussion and answer some readers’ questions. The responses can be found in the latest HairClone article on the main page.
银行ser有一些不同的意见vice, yet virtually all readers agree that HairClone’s proposed dermal papilla injections is a subject of high interest. HairClone can begin injections once it has funded a GMP certified system to produce the product.
Regarding The Latest Dr. Tsuji Talk (8/7/19)
Dr. Tsuji has stirred up conversations around the internet over the past few days. Readers have been posting Youtube videos in recent comments, threads are going up on Reddit, and message boards are discussing factors of Dr. Tsuji’s potential hair multiplication therapy. Due to the fact that most of the information in question was posted in the Japanese language, misinterpretations have began to pop up. I’ve done some digging on this subject and will do my best to (partially) clear up the current Tsuji discussion here.
For starters, there is a Japanese Youtuber name “Youngjet” who posted avideowhich shows him going to a conference in Japan and meeting Dr. Tsuji. There is also another Japanese blogger Tonegawa who posted aninterviewhe did with President of Organ Technologies (Tsuji’s company) Yasuhiro Sugimura. I’ve spoken to both bloggers in the last two days.
After this Japanese content started to spread and discussion grew there has been a post on Reddit which claims Tsuji’s clinical trials were ‘already done’ in 2019. I asked Youngjet who met Tsuji a month ago about this and according to him, this is false. Excerpt from our conversation:
FT: Have human trials already begun?
Youngjet: No haven’t. Not done yet.
Youngjet believes the Organ Technology treatment may enter the market in 2020/2021 and have a price tag of 20-40 million Yen. (roughly $300,000) This would seemingly put it out of reach for the majority of the public until the price decreases due to competition and technology improvements.
I then spoke to Tonegawa who interviewed the President Sugimura of Organ Technologies. Surprisingly, Tonegawa believes that the treatment will reach the public in 2024. He also thinks the trials will take about 2 years to complete.After further review, we are still left with a discrepancy between the two Japanese bloggers who had interactions with members of Organ Technologies. I will continue to update on this subject as more information is released, preferably from the company itself.
In summation: Confirmed that clinical trials have not begun yet. Discrepancy between beliefs that the treatment could reach the market between 2021-2024. Price will be between 20-40 million Yen (~$300,000) when the product does reach the market.
HairClone Offers Follicle Banking (8/2/19)
HairClone was back in the news today to announce regulatory approval of its hair follicle banking services. The UK startup intends to store patients’ hair follicles and one day offer cellular injection treatments to patients by utilizing the stored hair follicles. An interesting announcement from HairClone was that a first patient had banked their hair follicles – and it was a member of the company!
The biggest question everyone has about the treatment is how well will the injections work? That is an answer of course that time will tell. Between members of the company itself who will receive injections and patients close to Dr. Bessam Farjo who are willing to try the expanded DP cell therapy, it seems that there will be no problem reaching a sufficient number of initial case studies. Full story on the Articles page.
Transplant Surgeon Trialing New Injection Therapy (7/31/19)
一个新的播客采访“Tressle分享ss” forum of Reddit.com featuring long time hair transplant surgeon Dr. William Rassman. The interview was informative and discussed all typical things in hair restoration including minoxidil, finasteride, different types of hair transplants, and how to choose a good doctor for hair transplant surgery.
Interestingly, at an hour into the interview, Dr. Rassman mentioned that he was involved in his own hair growth research involving a specific set of proteins which have shown to cause upregulated hair growth in humans. For example, these proteins were associated with hair growth that accompanies facial moles. Dr. Rassman is planning to inject these proteins into the scalps of patients in a pilot study soon. You can listen to the snippet of the interview below which is set to start at the 1:00:04 mark.
PTD-DBM Drug Update (7/30/19)
One of the more interesting research developments from the past two years, the PTD-DBM research from Dr. Kang-Yell Choi of South Korea has emerged with an update. The research was mostly focused on stimulating hair regrowth and wound-induced follicle neogenesis. After about a complete year of silence Choi has decided to release some information regarding the development of his research into a new drug. We briefly discussed the topics of pharmaceutical partners and clinical trials in a Q&A session. Full article is on the main page.
Ultimate Guide Pipeline Update (7/27/19)
The Ultimate Guide gannt chart has gotten a full overhaul for August 2019. We saw 2 therapies leave the list including Fidia Pharma and Allergan’s setipiprant. Fortunately, there are about five new therapies which have been added. See for yourself on theUltimate Guidearticle and below.

Stemson Talks Human Trials (7/26/19)
Yesterday, it was announced that Stemson Therapeutics does indeed have an aim at a date to start clinical trials. This is an important news development and now theoretically puts them ahead of Rapunzel who has no indication of impending clinical trials. Head over to the articles page to read up on where they are at and join the discussion. Many readers are beginning to evaluate just which company is ahead of the game when it comes to hair multiplication therapies.
New Discussion On Current Events (7/24/19)
After several updates from major hair companies have come out over the past two months it leaves us at a rather interesting time period. The current focus and landscape of future treatments is starting to become a bit unclear. A few questions are beginning to be raised for Shiseido, also with the focus shifting onto the hair multiplication companies it now becomes a question of which approach is most likely to work. I’ve created a new thread to discuss these things as we wait to hear more updates and dish out small details that won’t get a whole update here. Check it outthe new thread here.
THTC & Instant Hair Sponsors (7/24/19)
The well known and respected Drs. Robert Jones and Jonathan Huber of theToronto Hair Transplant Centrehas become a sponsor of Follicle Thought. He’s also put up a banner for his hair fiber productInstant Hairwhich cosmetically thickens the appearance of hair. Dr. Jones was one of the first doctors to study PRP injections
Poietis Hair Bioprinting Update (7/22/19)
One of the most high profile hair follicle collaborations in the world is back in the news. Just about 3 years ago L’Oreal and Poietis embarked on a research agreement to create a bioprinted human hair follicle. This would be an unprecedented task for the hair industry and the tissue engineering industry as a whole. I received feedback from a top executive at Poietis that things have been going well for the hair follicle project and the company may have some big news to announce in the next few months. Head over the articles page for full details and commentary from Poietis.
NGF-574H Product (7/20/19)
我也e been following the development of this product for over a year now and sharing news as it has become available.我认为做一个与公司售前to help make the product available to the readers who wanted to try it, however I’ve decided otherwise. To sum it up: the product earned rightful attention due to its good background resume (stem cell therapeutic company, government funded R&D) and has shown positive preliminary data. However, I was expecting the product to launch with a full data set available, and without one there’s not enough evidence to get involved. I will remain neutral and plan on observing the reaction of its Korean release through NGF social media over the next several months before any further updates.
Media Article About Turkish Drug Identified (7/17/19)
An article published today on the Turkish news website Haberturk.com describes a new hair loss drug which is being put through clinical trials in Turkey. This has lead to readers sending the link to share with me. Here’s a translated excerpt from the article:
“A molecule that can be used in the treatment of hair loss and a drug that can be used in the treatment of baldness have been developed by the Turkish company which continues its activities in the USA.”
起初,听起来有趣,我觉得它may represent a new drug to be added to the landscape of potential hair growth therapies. However, upon further review it turns out that the drug this article is referring to is none other than SM04554 from Samumed which has been involved in a late stage trial in Turkey since last November.
Histogen Part 2 + Mexico Trial Update (7/16/19)
The second part of the Histogen review is now published on the articles page. It is undoubtedly a story like none other in the hair growth industry. HSC has produced the most appealing images of hair regrowth that we have seen thus far, yet over the past ten years involving many ups and downs, it has not managed to progress far in the clinical trial process. Also, I have received an update from Gail Naughton of Histogen about the status of the late stage HSC trial in Mexico. Visit the main page to get the full news.
A Deep Dive Into Histogen’s History (7/12/19)
I thought it was an appropriate time to take a look at one of the most iconic companies in the history of modern hair restoration. From legal battles to overseas clinical trials, this story has it all. The only thing it’s missing (for now) is the happy ending of a market release. But it’s been a long time since Histogen’s first clinical trial in Honduras and I thought that it would be a fun trip to revisit the story. There’s been a good reaction from the readers so far. Check out the latest article on the main page now.
RT1640 To Treat Permanent Chemo Alopecia (7/8/19)
A new article has been posted displaying an impressive result by RT1640 regenerating a lot of hair for a middle-aged woman who had experienced permanent chemotherapy-induced alopecia. Women who experience permanent hair loss due to use of the drugs Taxol and Taxotere currently have no options to restore their hair. RT1640 shows in the photo evidence it has the potential to treat PCIA and can be classified as an orphan drug to the US FDA which is useful to any company would want to further develop it.
Anti-DHT Shampoo Sponsor (7/7/19)
Hair Restoration Laboratorieshas begun sponsoring an advertisement on Follicle Thought viewable in the right sidebar. The company makes a product line including two shampoos, conditioner, and a minoxidil topical all containing a thoughtful blend of natural DHT blocking agents. I was informed the founder of this company began the pursuit of a well formulated shampoo due to his own struggles with hair loss.
RiverTown Photo From April (7/7/19)
It’s been such a busy past 2 weeks in the hair growth industry and that potentially means there may be a break in the news for a little while. I thought this was a good time to review some data from RiverTown Therapeutics’ drug RT1640 which was shared on Follicle Thought back in April. There are no other topicals right now claiming to regrow hair in a bald area of a 60 yr old scalp. Perhaps, the companies working on hair follicle replication are the only ones who could present a result like this, but truthfully most of those technologies are still years out in the development phase. RT1640 exists now.

What do you guys think of this result and where does this potentially put RT1640 in terms of strength against the other topicals of Breezula, Samumed, and Aclairs? I’ve started a discussion thread to talk about these things, please visit and voice your opinion ->RT1640 Discussion Thread.
Aclaris’ ATI-502 Misses Goal In Clinical Trial (7/5/19)
Among the barrage of positive news in the hair growth industry, there was some news from a well known company, Aclaris Therapetics regarding one its drugs which did not meet its clinical endpoints in a phase 2 trial. The drug, a topical JAK 1/3 inhibitor called ATI-502, was being trialed in subjects with alopecia areata over a 6 month period. During the trial, 129 subjects total applied ATI-502 to their scalps twice daily.
At the end of the 6 months the primary endpoint was evaluated. In this case, the primary endpoint was the mean percent change from baseline in the Severity of Alopecia Tool (SALT) score. Secondary endpoints included change in Alopecia Density and Extent (ALODEX) score and multiple investigator and patient reported outcomes. As these endpoints are tallied, the subjects who were receiving the topical JAK inhibitor ATI-502 are compared to the subjects who received just the “vehicle” which is usually a substance like salt-water.
In this case, the vehicle performed in a similar range to the actual drug candidate thus the ATI-502 program for alopecia areata will be cancelled. What’s interesting is just recently, Aclaris announced positive results using topical ATI-502 to treat a more common form of hair loss, androgenic alopecia. While Aclaris initially seemed optimistic about moving ATI-502 into a further phase 2 for androgenic alopecia one would have to thinkthey may be second-guessingthat strategy now due to the areata trial. If that was the case, the list of topicals in trial for AGA would be shortened to Samumed and Follicum.
Stemson Therapeutics Unveiled (6/29/19)
Earlier this week I shared the news of Stemson Therapeutic’s new website being launched through a Sanford Burnham Prebys press release. The news is quite exciting as Alexey Terskikh and the team at Stemson believe they have figured out a solution for getting multiplied hair follicles to grow through the skin surface correctly. The Stemson website also just shared details of a multi-million dollar investment from pharma company Allergan. With sufficient funding in place, the progress and development of Stemson’s hair regeneration treatment should be able to progress at a steady pace.
This Just In: Norrin (6/27/19)
It’s been quite a week for hair follicle research, one of the busiest I’ve ever encountered actually. Among other news releases, a scientific journal article was published detailing anew proteincalled “Norrin” which positively affects follicular keratinocytes and enhances hair growth.
The study was published in the STEM CELLS journal and was lead by Daniel Aberdam PhD of INSERM and the Université de Paris. In the research it was discovered that dermal fibroblasts分泌细胞外囊泡(EVs)扮演important role in cell to cell communication. The EVs then stimulate dermal papilla cells to secrete the Norrin protein, which then in turn activates follicular keratinocytes. Norrin was also shown to play a role in WNT/beta-catenin signaling which is known as the predominant cellular pathway responsible for the control of hair growth.
Big News From Christiano & Rapunzel (6/26/19)
Angela Christiano and company at Columbia University have achieved new degrees of success for creating 3D printed hair follicles. It seems that the research team has finally found a way to create a successful environment in which to culture dermal papilla cells to create the formation of hair follicles. Columbia University has even created a nice little video describing the process which is featured in the latest article on the Follicle Thought main page.
Ultimate Guide Quick Update (6/21/19)
The Ultimate Guide to Hair Regeneration has been updated with a few minor changes. Some companies have moved up in their positions due to recent news announcements and data releases. More updates to come regarding these companies soon!
Aclaris Results Photos! (6/17/19)
Main page article has news about Aclaris’ latest press release on positive results from its trial on AGA. More importantly, this Follicle Thought article contains photos of the trial results and information about Aclaris improving its dose concentration for its next trial in AGA.
Subscribe To New Follicle Thought Youtube Channel! (6/15/19)
Hi all, please visit the newFollicle Thought Youtube Page hereand hit the red subscribe button on the right hand side! I’ve just uploaded a new video featuring the Celino/Medipost NGF-574H product which will be launching in South Korea next month. You can find this video on my Youtube channel.
Follica Announces Positive Data (6/13/19)
Head over to the Articles page to read about Follica’s latest announcement concerning data from an ongoing safety and optimization study they have been completing, commentary from Follica execs, and plans for phase 3/pivotal study for FDA approval coming soon!
New Company Applied Biology (6/11/19)
A new company developing solutions to alopecia has emerged onto the scene recently. Applied Biology is headed by renowned hair researcher Dr. Andy Goren and has several other key personnel on board. The company has 7 treatments in development for alopecia currently. Read about them more in-depth on the Articles page.
Aclaris Update (6/819)
The articles page has a detailed update from Aclaris’ recent presentation at the Jeffries Healthcare Conference in New York City. CEO of Aclaris Neal Walker mentioned that a report on the JAK inhibitor trial for androgenic alopecia will be coming out soon.
Support Follicle Thought (6/6/19)
Please visit the donation page for Follicle Thought and make a monthly or one-time contribution. A lot of people tell me how much they appreciate the work that is done here and I would certainly appreciate it also if you would be willing to make a monetary contribution to this site for all of the time and effort it takes to manage. Please click the image below and contribute.
Histogen Presentation (6/4/19)
A new article with a Histogen presentation has been added to the articles main page. Histogen has a new CEO and discusses things like the female hair loss trial, male hair loss trial, and a potential topical HSC in the video. Have a look.
UCLA Starts Hair Growth Company (5/29/19)
Lactate promoting drugs are now being developed by a UCLA spinoff company for the purposes of growing hair. This is related to the research that made headlines in late 2017. The name of the company and more details can be found in a new article on the main page of Follicle Thought.
Follicum BioStock Presentation (5/28/19)
A new video presentation with discussion from Follicum CEO Jan Alenfall has been added to the articles page. Alfenfall talks about the new topical formulation for FOL-005 as well as plans for a new clinical trial.
Replicel Announces New Hair Trial (5/21/19)
In a May 2019 Update newsletter, Replicel has announced a new trial for hair loss involving its dermal injector. The update reads as follows:
“Over the next 18 months you should expect to see:
- new clinical data from a study completed showing the value of the dermal injector in treating hair loss, and
- clinical data from the Shiseido-funded study of our hair loss treatment (RCH-01) in Japan with clarity around its intentions to launch the product.”
This clarifies the difference between the Shiseido study and the dermal injector study. I hope to receive an update from Lee Buckler with more details on the injector study soon.
Shiseido Results Coming Soon (5/20/19)
Article has been released on the main page detailing an email that was reportedly sent by the lead investigator of Shiseido’s RCH-01 trial. Check it out for full details.
Rivertown New Angle Photos (5/14/19)
The main page has been updated with some new exclusive RiverTown Therapeutics photos showing a 60 yr old man’s 6 months results using RT1640. The results are impressive and display thick regeneration around the crown ridge of the man. Check them out.
Follica News (4/28/19)
Follica has shared news about its clinical trial pathway and more in a 2018 Annual Report. An article has been shared on the main page which details Follica’s current study and contains tidbits about furthering its pipeline for hair growth, check it out.
Leonhardt Ventures Discovers Sonic Hedgehog (4/25/19)
The company working on the “HairCell” technology has added another electrical signal to its repertoire, the sonic hedgehog protein. Leonhardt Ventures announced the discovery viaa press releaseearlier this month. The HairCell technology is composed of a helmet – containing electrodes – that send bioelectric signals to the scalp to release regenerative proteins. Sonic hedgehog protein has been known for many years to be a powerful protein to stimulate hair follicle regeneration. By adding this signal to the HairCell package, Leonhardt Ventures hopes to create a formidable hair growth technology. The HairCell technology already includes bioelectric signals for proteins such as PDGF, VEGF, IGF-1, etc.
HairCell is currently engaged in a clinical trial taking place in South Africa.
Cassiopea Announces 12 Month Results (4/16/19)
New article is up sharing Cassiopea’s 12 month results from their phase 2 dose ranging study. Spoiler alert: the results were good. Check the main page to read about a new possible alternative to finasteride named Breezula. The article includes information on a phase 3 trial and a new POC study for Breezula in women.
New RiverTown Photos (4/15/19)
一个新的文章发布到主页沙ring new RiverTown photos of a 14 month hair growth result and also a before and after comparison of pigment restoration. Both photo comparisons are impressive in my opinion. RiverTown is continuing to look for a partner to advance its clinical trial progress so that the treatment can actually be made available to consumers. Please share the new article and post a comment of what you think of the photos.
Medipost Update (4/2/19)
Medipost has recently shared an update about their hair growth product NGF-574H. The topical product will now be released under a new company name “Celino” and is scheduled for a July release in clinics and hospitals. Full details are found on the main articles page.
HairClone Featured in Article (3/21/19)
HairClone was featured in DailyMail online magazine earlier this month. Along with giving a full rundown of the HairClone procedure and follicle banking system, the article also mentions a price of £2,500 to extract follicles from a patient and place them into cryopreservation. The cost would then be £100 each following year to store the follicles in the deep freeze. A price for injections of cultured hair follicle cells has not been disclosed yet and Dr. Bessam Farjo is quoted in the article to say“The specific figure is currently very proprietary information but what we can say is it would be comparable with premium hair transplant procedures which can cost several thousand pounds.”
Click here to read the full DailyMail article on HairClone.
Rapunzel Surfaces (3/21/19)
Angela Christiano’s hair multiplication startup Rapunzel finally has a website. Apparently, the company will work on both hair and skin regeneration. The link to the website and more information is found on main article page.
Replicel Newsletter (3/9/19)
Replicel published a newsletter earlier this week on March 5th which detailed some of the company’s current initiatives including its skin and hair programs. The article is on the main page mentions Replicel’s anticipation of a Shiseido announcement soon now that the trial has completed.
Trinov Giveaway Ends Today (2/26/19)
The winner of the male Trinov lotion giveaway will be announced today. If you haven’t already commented to be entered into the contest, please do so by the end of the day.
Follicum Develops Topical + Interview (2/7/19)
Follicum has recently announced news of results from an in-vivo testing of their new FOL-005 cream topical. The tests in mice showed favorable results comparable to an industry standard hair growth drug. More details and the link to the press release are up on the main articles page.
One day later, Follicum has also released a new interview with Maria Ekblad Director of R&D at Follicum. Within the interview, Maria discusses topics such as the upcoming human trial for the topical FOL-005 and Follicum’s plans to create a partnership deal to further develop FOL-005 into the consumer market.
Interview With Follicum’s R&D Director Maria Ekblad
HairClone Interview Part 2 (1/30/19)
The second installment of the interview with Paul Kemp PhD of HairClone is up on the Articles page. Paul gives a very detailed account of where HairClone is at in their development process and how soon the treatment could become available to patients in the UK
RiverTown Gray Hair University Research (1/30/19)
RiverTown Therapeutics Inc.has completed preliminary research with University of Alabama at Birmingham to test the ability of RT1640 to reactivate melanocyte stem cells in mice. These are the stem cells which give hair its pigment. Read more about it on the Articles page.
Trinov Review Article Updated (1/14/19)
The latest Trinov article on the main page has been updated with a full list of Italian pharmacy websites selling Trinov. I’ve also put out a request to all readers using Trinov who are also documenting their progress with before and after photos to share the photos for posting on Follicle Thought. Photos can be edited to maintain confidentiality. It would be a big help to the other readers.
In addition, some discussion has started bubbling in the comments from readers who are using Trinov. Please share the article where you think it would be useful. Thanks
Follicum Enters Research Collaboration With The University Of Bradford (1/1/19)
In mid-December late last year Follicum announced it entered an agreement with the Centre for Skin Sciences (CSS) at the University of Bradford UK to investigate the mode of action of its peptide for hair growth FOL-005. The CSS will be using in vitro experiments combined with bioinformatics data mining and big data analysis to learn more about how Follicum’s peptide works.
The goal of the project is to achieve a better understanding of how FOL-005 drug works to initiate hair growth which should strengthen industry partner opportunities for the company. This collaboration will also investigate Follicum’s peptide class which is being developed to treat diabetes. Interestingly, Dr. Kevin McElwee (CSO of Replicel) is one of the researchers at the CSS who will be involved in the project. The news was published in a press release by Follicum and then reiterated in a longer year-end news wrap from the company titled “A Christmas message from Follicum.”
Click here forA Christmas message from Follicumand anews releasefrom the University of Bradford.
New HairClone Interview (12/24/18)
Fresh HairClone interview up on the Articles page. Merry Christmas everyone!
Thank You For Your Support (12/23/18)
Sincere thanks to all of my readers who visit this website frequently and all who share my articles with others. It is great to be able to make a positive difference in so many people’s lives through this work.
As you can imagine, I put in an enormous amount of hours throughout the year to make this site the best it can be and to provide you all with consistent information on hair growth solutions which will improve our lives. At this time I’d like to kindly ask that all of you who appreciate the service that Follicle Thought provides to visit my Patreon donation page and make a one-time donation or a recurring monthly contribution. It is much appreciated. Please click the image below to make a contribution to my donation page.

HairClone AMA On Reddit (12/14/18)
Paul Kemp, CEO of HairClone, has started an “Ask Me Anything” thread on the discussion website Reddit. Paul and the team at HairClone are working hard to develop an innovative procedure to rejuvenate, and hopefully one day, fully regenerate new hair follicles. I’ve noticed some troll comments in his discussion. It would be nice for those of us who appreciate HairClone’s work to head over to the discussion and show some love to Paul and the team! If interested, please go show your support and add a comment for a company who is working to help you.
New HairCell Video & Clinical Trial (12/12/18)
The Leonhardt Ventures startup HairCell has recently updated its website to showcase a new video demonstrating its technology being applied to a patient. The video was filmed at a hair clinic in South Africa where a clinical trial for HairCell is currently underway. For more information and to watch the video check out the latest HairCell article on the main page.
Cosmetics Business Article (12/6/18)
A new article has been published on the main page which contains an exclusive PDF to the Cosmetics Business Magazine November Trend Report. I was featured in the article and provided information for Cosmetics Business’ article.
Fidia Announces Trinov Arrival (11/29/18)
Today, Fidia Pharma of Italy announced that Trinov has arrived in pharmacies in Italy. I imagine this means that Trinov is now or will immediately become available for purchase online.
Trinov Website Launches (11/23/18)
I just received an email sharing a new Trinov website:TrinovAnticaduta.com.This one does appear to be an official website for Trinov. The page is in Italian and can be translated, it mainly features a sign up form to download the “Trinov ebook” for either the men or women’s version. I downloaded the men’s Trinov ebook, however it is a pdf in the Italian language and cannot be translated. There is also a short YouTube video for Trinov featured on the page. Fidia Pharma’s logo is at the bottom of the TrinovAnticaduta page and for good measure I looked up the domain on Whois and saw that the page is registered to Fidia. Things will just keep getting more interesting from here.
Happy Thanksgiving + New Article (11/22/18)
Wishing a very warm and Happy Thanksgiving to all of my readers in the US. I am thankful for all of my readers who visit this site daily and support Follicle Thought in various ways.
Working On Hair Growth? (11/16/18)
2022世界杯附加赛预测英格兰vs美国皇冠比分卵泡认为感兴趣的联系scientists and startups who are working on hair growth or hair loss prevention research. If you or a colleague is working on hair growth please get in touch on the世界杯2022入围名单 page, even if you are not ready to share news on the site yet. Follicle Thought can offer networking and other resources to benefit your development. Thanks
HairClone Announces Crowdfunding & Paul Kemp Comments (11/14/18)
Late last week, HairClone officially unveiled its crowdfunding campaign with the Euro-based crowdfunding company Capital Cell. HairClone is offering equity based crowdfunding, which means anyone who makes an investment (£500 minimum) owns equity in the HairClone company. Full details on the campaign are listed in the latest HairClone article on the main page of Follicle Thought.
Dr. Paul Kemp, CEO of HairClone, has began responding to readers’ questions below the article. His responses are lengthy and provide deeper insight to HairClone’s background and what differentiates the company among others in the cellular hair cloning industry.
New Medipost NGF-574H Info (11/7/18)
A new article has been posted today featuring an expanded presentation of the stem cell-derived cosmetic product NGF-574H. This product comes from Medipost of South Korea, a company focusing on cord blood stem cell therapies. The NGF-574H product is an interesting one and I hope you will enjoy viewing its pdf presentation in the latest article.
Follicum Phase 2 Data With New Interview (11/2/18)
Earlier this week I shared the announcement of Follicum’s phase 2a scalp trial results. Since then, Follicum has uploaded a new interview with CEO Jan Alenfall which discusses the findings from the phase 2a trial in greater detail. The link and highlights to the article are in the latest Follicum article on the main page.
Proactive Readers Comments (10/29/18)
I feel inspired to share on the Updates thread a few uplifting comments I received from readers in the past week. Following my admonition to a previous commenter that sending out short, supportive emails to hair growth companies (without asking for extra information that you know they are not ready to share) would be a worthwhile endeavor and boost morale, I received some encouraging responses from two frequent visitors of this site who followed through on the idea. Their comments are shared below.
From “Welsh Dragon”:
“Re the post 10/17/18 I really liked your response to the request made by one of your readers to email companies asking for information. So I decided to do what you suggested and sent emails to Organ Technologies, Rivertown and Follicum (no particular reason) basically thanking them for their work and wishing them success with their products. Interestingly I received a very nice response from Organ Technologies which really surprised me. Correct me if I’m wrong but I think the sentiment is to be thankful and encouraging to what is being done appose to being negative that we have yet to get what we all want.”
From “Hope”:
“I also reached out to Histogen and Follicum a few weeks ago as well thanking them for all their hard work in bringing a safe and effective treatment to people all over the globe with hairloss issues and expressed how much we all value these companies. I held back from asking about market release as you had suggested. I received a very nice reply from Histogen.”
I think we can all agree that the little things do make a difference in our lives and I’m thankful to hear these responses from my readers.
Regarding New Trinov Site *Updated* (10/24/18)
Update:I have been in contact with the administrator of the new Trinov site. It’s apparently a European based site, it is not officially affiliated with Fidia Pharma. The admin plans to use the site to focus on Trinov reviews and vet the product. All in all, it seems benevolent. For the record, we have known for a while that TrinovCapelli.com is a domain which is owned by Fidia Pharma and expected to be the official Trinov website. As of now that domain is not active yet.
Due to the concern of several readers, I’ve removed the link to the new Trinov website that has popped up on the net. For now, the website only contains an email address subscription box which really poses no issue to anyone who subscribed. At this time, it’s not confirmed who the actual owner of the new Trinov site is, so use your discretion until we find out more regarding this matter. Until more information is known the website will not be shared on Follicle Thought.
Positive Signs For HairClone (10/22/18)
Certainly a company with a unique background and game plan, HairClone has received some recent media attention. The UK biotech startup was featured in this month’s publication of “Consulting Room” online magazine. In the article both Dr. Bessam Farjo and Paul Kemp of HairClone provided insight as to the company’s current progress and plans for the future. Things seem to be coming along well for HairClone and we should expect to hear from them again within the next several weeks. Full story is on Articles main page.
Transdermal Delivery Vehicle (10/22/18)
Prometheon Pharma is open to research collaborations using their transdermal delivery technology with hair growth formulas or for other drug delivery applications. If interested in discussing an opportunity pleasecontact them.
Response To Recent Comment (10/17/18)
Recently, a reader of this site asked me via comment:
“I like to know if one of the things that Follicle can do is to right an e-mail or letter, in our as a community (people who are visiting FT website) name, to the first four companies to let them know that they are lots of people who are waiting for their products, and a cure for hair regrowth and hair loss and ask them to give as the update as soon as they can, and release their products asap.”
This was certainly a fair and genuine comment. It prompted a response from me that I would like to share here again to hopefully be an inspiration and help us find that gratitude during the periods of waiting. My response was:
“if I thought that would motivate the companies to do something different than what they are already doing… to speed-up the release of their products, I would. However, I am confident they already understand (we are waiting).
One thing I have recommended in the past is for readers themselves to send short, thankful emails to these companies expressing your gratitude for what the company is doing to create a hair growth treatment and that it means a lot to you. Asking nothing in return (such as release date or update on progress.)
Imagine how it would feel for you at your job to receive 4 emails throughout a day telling you how good of a job you are doing and how appreciated you are, versus receiving 4 emails a day asking when you will be finished and expecting a response. Could that potentially affect your overall productivity and mindset?”
Eczema Drug Shows Efficacy in Alopecia Areata (10/11/18)
Carrying on in the tradition of JAK inhibitors, another FDA approved drug, dupilumab, has proven to be useful for the treatment of the auto-immune disease alopecia areata. A case study was published yesterday in theJAMA journaltitled “Hair Regrowth in a Patient With Long-standing Alopecia Totalis and Atopic Dermatitis Treated With Dupilumab.”
Dupilumab is FDA approved for treating eczema aka atopic dermatitis and sold under the brand name Dupixent. As the story goes, a patient with alopecia totalis (a form of areata which leaves a person’s head completely bald) was being treated for eczema by the drug Dupixent. After 6 weeks the patient first began to notice progress in terms of hair growth and at 7 months she had noticeable pigmented hair growth on her scalp. Notably, the patient stopped taking the dupilumab for a period of time and noticed her growth subsided; when she began taking the drug again the hair improved once more. This provides another useful therapy option for patients seeking treatment for AA. One would imagine a topical version would be worthwhile to investigate.
Shiseido Data Next Year (10/10/18)
As promised, I am providing an update on the highly anticipated development of Shiseido and RCH-01. This may not be the exact update everyone was hoping to hear, but nonetheless, progress and continued development are what we need to succeed. The update in summary is this: according to my source we will be getting the data from Shiseido’s trial of RCH-01 in 2019. There is no scheduled date for the data presentation, but I anticipate it would be within the 1st quarter of the year.
I know we would all prefer to hear the results this year, however with the good news of Trinov coming by December we have a lot to be thankful for and look forward to. Stay tuned for more good news.
Follicum To Reveal FOL-005 Results Soon (10/4/18)
We will be hearing phase 2 clinical trial results from Follicum, not only by the end of this year, but by the end of this month. The main Articles page has a link to Follicum’s latest news announcement. We wish them all the best for their data rollout.
Fidia Looking At Other Markets For Trinov (9/28/18)
Fidia has shared the English version of the Trinov press release on their website and it contains a small update about the company’s plans for expanding the commercialization of the product. Check the main page Trinov article to see the available details.
Fidia Pharma Announces Trinov Release (9/25/18)
这最终还是发生了。检查的主要文章page to hear the latest details about Trinov aka the Brotzu lotion. Fidia has announced via press release that the anticipated product will indeed be released by the end of the year. I am extremely happy for this announcement, let’s see how this goes.
Recent News Round-up (9/24/18)
Below are some of the latest new items that have been making waves around the internet lately:
NTU Working To Prevent Chemo-Induced Loss –Researchers from the National Taiwan University have developed a model for preventing chemotherapy induced hair loss, according to their publication in Cancer Research journal. The team, lead by professor Lin Sung-jan, identified a specific type of cell that hair follicles utilize to compensate for the toxicity which occurs during exposure to ionizing radiation (chemotherapy). These cells are called transit-amplifying cells (TAC). Preclinical animal testing with applied TAC-derived progenitor cells showed a 70-80% reduction in hair loss after chemotherapy and radiotherapy. Importantly, Sung-jan hasrecently statedhe is in talks with companies about conducting trials on humans. It’s interesting to note thatLin Sung-janhas done an extensive amount of research on hair regeneration in the past. Hopefully this treatment could potentially be used for more common types of hair loss as well.
Pfizer Reports Positive AA Trial Results –A JAK3 inhibitor and a tyrosine kinase inhibitor (TYK2/JAK1) have shown statistically significant results in a phase 2a trial conducted by Pfizer. The company announced the results on September 15, 2018 at the European Academy of Dermatology and Venerology Congress. Subjects of the trial received oral doses of the drugs over a 6 month period. The TYK2/JAK1 inhibitor showed the greaterefficacy, improving hair regrowth by 49.5 points on the Severity of Alopecia Tool scale, comparedto an improvement of 33.6 points by the JAK3 inhibitor. However, Pfizer hasapparentlydecided to move forward with its JAK3 inhibitor due to 2 adverse events in the TYK2/JAK1 inhibitor cohort during the trial. Pfizer’s JAK3 candidate, PF-06651600, was also recently grantedBreakthrough Designationfrom the US FDA for treating alopecia areata.
“Smelling” Receptor Keeps Hair Growing –Many of you may have noticed the headlines regarding sandalwood and hair growth over the past week. The research everyone is talking about comes from Ralf Paus and his team at the Monasterium Laboratory GmbH. For the record, Paus is also the main researcher behind the WAY-316606 hair growth discovery. This time Paus et al identified an olfactory receptor in hair follicles, OR2AT4, which plays a role in regulating hair growth or inhibition. Olfacory receptors are responsible for detecting odors in cell membranes and provide the basis for our sense of smell, they do carry out additional functions though, as demonstrated by Paus.
This particular research which was published in theNature journalshowed that a synthetic version of sandalwood, called Sandalore, binds to the OR2AT4 receptor in hair folliles and prolongs their anagen (growth) phase. The hair follicles studied were treated in a petri dish. Paus has subsequently announced that a completed clinicaltrial20使用局部版本的女性志愿者Sandalore showed a reduction of daily hair loss. There is also another larger clinical trial ongoing now which hopes to confirm the effect and announce results in early 2019. Paus has gone as farto say“there is even a chance that this might be able to bring the hair back.” We’ll keep our fingers crossed.
I believe it’s likely that more so than sandalwood or Sandalore, targeting the OR2AT4 receptor with a newly designed molecule may someday prove to be a truly significant therapy. Until then, a little Sandalore based hair care product in your regimen probably couldn’t hurt.
Oxy133 Crowdfunding Campaign Successfully Funded (9/13/18)
Last month, I shared a crowdfunding campaign byMAX BioPharma.The company launched the campaign to raise money for a preclinical hair growth experiment in mice with their lead compound Oxy133.
There was a important campaignupdatemade by MAX BioPharma founder Dr. Farhad Parhami on August 29th stating that due to changes in the company’s other research programs, the budget of the hair growth experiment would be reduced significantly. This brought the campaign goal of $5,000 down to $2,900.
We’re happy to report that as of today, the campaign has been fully funded and MAX Bio says they will begin the trial in the next couple of weeks once the money has been transferred. Here’s a quote on the potential of Oxy133 by Dr. Parhami which may be of interest:
“Curis (now-dormant company) had performed a lot of studies on targeting the Hedgehog pathway for hair growth with very promising results, however, their compounds caused orthosteric activation of the pathway (turning it on everywhere and robustly which is not safe) vs. Oxy133 which causes a much more regulated and limited allosteric activation of the pathway only in stem cells. This could make Oxy133 a blockbuster. Let’s see what happens.”
Brotzu Coming Soon? (9/11/18)
There have been recent discussions on Italian hair forum websites that indicate the Brotzu lotion may very well be moving ahead and preparing for imminent release. After commenter “Ahmed” brought it to my attention, I went back to check the Bellicapelli forum (the site which had the information on the Brotzu presentation at the Sitri Congress in April). I found a response from user “carlitos71” on thispagewhich seems to display the new theories on the Brotzu lotion.
To summarize, a member of a Italian hair forum posted alinkwhich displays a trademark name “Trinov” filed by Fidia Pharma. The assumption here is that Trinov is the official product name of the Brotzu lotion.
Also, the website URL Trinovcapelli.com has been registered by Fidia Pharma. This is shown on awebsite verifier service.Capelli means hair in the Italian language.
So, a new trademark filed and a website registered by Fidia Pharma. Although a disclaimer that this news is not official yet is warranted, this does appear to be very strong evidence that the Brotzu lotion is coming soon. The hearsay on the Italian forums is that the product is intended to be released in October 2018. Ciao for now.
Feel Good Friday (9/7/18)
I hope you’re having a good Friday. While I am a bit surprised that out of the thousands of people who visit this article every week, still not one person has commented with an idea or practice that they can do to help hair growth treatments succeed, I feel the need to share some upliftment today. It’s coming from the original contributor of Feel Good Friday himself, Deion Sanders. A while back I shared a video of Deion getting his second FUE transplant to thicken his hair. The results are starting to come in and Deion could not be more enchanted by his own hair-restoration miracle. For a guy who was basically NW7 before restoration, the result is impressive. Enjoy his sentiments below.
View this post on Instagram
The Part You Play Makes A Difference(8/30/18)
It is important for all people to receive reminders of encouragement and support. This is especially pertinent for those of us on the journey to receiving new hair growth treatments. I’ve decided it was the right time to share a few thoughts on this subject which I feel will be beneficial for many of us to hear.
As we wait and anticipate the market release of a new hair treatment there may be times when the waiting gets to us and we feel disappointed, frustrated, and even depressed. This is understandable. However, like many other times in life, a simple change of perspective can lift our mood and positively impact how we feel about a situation.我也e said it before and will say it here again, your gratitude for these potential treatments and the people behind them is vital and will brighten your day if you practice it on a regular basis. Make a commitment to gratitude. It’s especially important for those who are in the routine of logging on the internet and checking for news daily.
But, there’s an even more important step to take – your participation and contribution. The topic of “a cure for hair loss” or “a hair growth treatment”, for many of us, is one of the most important issues in our lives. I realize that question may not have previously crossed the mind for many. It’s here now. For a personal example, I’m not a scientist who creates molecules in a lab, so I decided I would organize the hair growth treatment news and spread awareness. It’s been a gratifying practice for me.
What is one thing you can do to help new hair growth treatments become a reality?Be creative. Your activity, whatever it might be, will give you a sense of empowerment. You will be contributing to the goal of new hair growth treatments becoming available in the world. Please share in the comments of this page your ideas or practices for how you personally choose to contribute to the success of new hair growth treatments becoming a reality. Remember, every idea or action is worthwhile and supports the outcome. Best wishes. Thank you
We Will See Results This Year (8/28/18)
Follicum has announced that their phase 2a trial has officially completed in Germany. Results from the trial will be shared by the end of the year, and may be here before winter. This is important as we will get a good understanding of Follicum’s hair growth drug FOL-005 from this trial. Link to press release is on Articles main page.
For Samumed, Phase 3 Is Official (8/21/18)
Yes, it’s happening. Head on over to the Articles page to read Samumed’s latest newsletter announcing the initiation of their phase 3 trial for hair growth drug SM04554.
New Hair Cloning Company Formed By SBP Research (8/17/18)
Alexey Terskikh PhD of Sanford Burnham Prebys research institute has news to share about his hair follicle research. The Articles page gives you all the highlights on what advancements Terskikh has made over the past three years and when he is planning to take his cloning method to FDA human trials. This is one example of a peer reviewed journal article which actually developed to human translation in a timely manner. Happy Friday
‘New Beauty’ Article On Hair Solutions (8/16/18)
的女性杂志最近新的美丽的特色veral prospective hair growth therapies in a print article. The feature contains several interesting and worthwhile anecdotes. Check the Articles main page to read about Dr. Cotsarelis’ new research on setipiprant for female alopecia, Histogen’s view on the number of injection sessions which may be necessary to get the most out of HSC, and more.
Crowdfunding For Hair Growth Study (8/10/18)
MAX BioPharma, a company working with Hedgehog pathway therapeutics, wants to test its lead compound for hair growth in mouse model. If data from this experiment turns out to be positive the company says it will reach out to cosmetic or larger pharma partners to commercialize the product. While we currently don’t know how this therapy will fare for hair growth, in the past we have seen impressive potential from stimulators of the Hedgehog pathway.
The crowdfunding campaign is registered on Experiment.com and MAX BioPharma is seeking to raise $5000 to complete the mice studies with their compound Oxy133. If funded, the company expects results by November 12, 2018. To visit the campaign please click the image below, and use your best discretion if considering a contribution.
Samumed Raises $438 Million (8/7/18)
San Diego-based Samumed has raised a whopping total of $438M in equity financing. The company reported this milestone in a press release via their website. CEO of Samumed, Osman Kibar PhD, detailed in the press release:
“我们现在在一个幸运的位置移动our later stage programs to commercialization, as well as expand on our earlier stage science and clinical portfolio.”
SM04554, Samumed’s topical for androgenic alopecia, is one of the company’s late stage programs. The other late stage program within Samumed is their drug for osteoarthritis. Phase 3 trials are very costly so the numbers here make sense for Samumed to be pushing forward. Thisnewswould indicate we should be hearing about Samumed initiating a phase 3 trial for the commercialization of SM04554 in the near future.
Researchers From India Create Artificial Hair Follicle (8/5/18)
University researchers at the Indian Institute of Technology Delhi have created a new laboratory hair follicle model which can be used to test drugs for hair growth effects. The team, lead by associate professor Sourabh Ghosh created a 3D human hair follicle model in a silk-based hydrogel.
The significance of this study is the new level of accuracy it could bring to the screening of drugs and compounds to induce hair growth. The current model used which has been used in the field for decades is the familiar “mice model” in which chemicals are injected or rubbed onto the back of shaved mice. If the substance gets hair to grow back faster than mice who do not receive the chemical, it is deemed that the substance holds promise for improving hair growth. We have learned time and time again, that substances which grow hair in mice do not always translate well to humans.Ghoshbelieves his new hair follicle model can provide a solution to this issue.
Source: Mediaarticleand peer-reviewedarticle.
Important Shiseido Update (7/31/18)
Just posted on the Articles page is an enlightening update pertaining to the availability of RCH-01 if Shiseido does indeed launch it to market. This would address questions of: how many people can receive the treatment in Japan? Can anyone in the world go to Japan for the treatment?
Continual Press Coverage For PolarityTE (7/28/18)
PolarityTE is a biotech company based in Salt Lake City, UT that kind of launched onto the scene late last year. Shortly after the company was formed it announced that its innovative lead product ‘SkinTE’ would be launching a limited release in several hospitals across the country. The launch of SkinTE came abruptly with no previous clinical trials for the product. This is due to the fact that SkinTE is based on autologous materials, which means they come from a patient and are applied back to the same patient. There is, of course, some manipulation done to the skin sample which is taken from a patient, but the FDA has deemed it to be minor enough not to need lengthy trials to reach the market.
我最近发表了一篇文章覆盖了一个我的故事n the press of SkinTE helping to possibly save the life of a burn patient (see Articles). In that post I shared an image from SkinTE’s website which shows an application for hair growth. What some may not be aware of is the fact that Dr. Denver Lough, CEO of PolarityTE, has done some legitimate hair follicle research while at Johns Hopkins University. Whether or not this will increase the chances of a “HairTE” product to become a success, we can’t say. However, it may be of interest to recall two peer reviewed articles that Lough and colleagues published involving the proteinsLGR5+andLGR6+stem cells and hair growth.
Gene Discovered Which Could Reverse Aging Factors (7/22/18)
More good research coming from the University of Alabama at Birmingham shows that a certain gene affecting mitochondrial function can dramatically reverse signs of aging in mice. The aging factors which were shown to be reversible include skin wrinkles, gray hair, and hair loss. Next comes the important phase where the researchers continue forward to translate this discovery to human use. Full article on the front page.
Big News From Histogen (7/17/18)
Histogen has announced a noteworthy development for its HSC product. Head on over the Articles section and read about it.
FDA Grants Fast Track For Aclaris’ JAK Inhibitors (7/9/18)
Aclaris Therapeutics has reported today they have received a “fast track” designation from the US FDA for their topical JAK 1/3 inhibitor known as ATI-502.
According to the press release, a fast track designation is“intended to facilitate the development of new therapies for serious conditions and with the potential to address an unmet medical need. A company with an investigational medicine receiving Fast Track designation may be eligible for more frequent communications with the FDA and may receive an expedited review of the new drug application.”
Press releasehere.
Perspective On Shiseido Timeline (7/7/18)
A new article on the homepage gives some insight as to when we may hear more from the cosmetic giant Shiseido regarding their trial of RCH-01. Said article contains the opinion of one industry figure who is close to Shiseido.
Histogen Recruiting For Female Trial in US (7/2/18)
Histogen announced today they are officially recruiting for a phase 1 clinical trial of HSC660 in female pattern hair loss. The trial will take place in Southern California and is for female particpants only, more details on front page.
Regarding Brotzu Lotion and Replicel Releases (7/1/18)
Today marks the first day of the 2nd half of 2018. It has long been anticipated, due to various reports, that both the Brotzu lotion and RCH-01 in Japan may come to market in H2 2018.To recap official announcements from these companies: In January 2017 Fidia made anenigmatic referenceto completing a product by the end of 2018. In 2016 Shiseido was veryconfident and vocalabout “curing baldness in 2018”, many of you will recall. (note that Forbes does not publish news based on “internet hearsay”)
Lately I’ve been receiving a few inquiries from readers about Shiseido and Brotzu release dates. So, I’m going to address the situation here and hope that this will be sufficient until more news comes from direct sources. I estimate that these companies would publicly address the release date of their products by the end of Q3 this year (end of Sept). As consumers we know there’s no guarantees for releases and if one or both of these products reached the market this year it would be a very fortunate situation. So, keep an eye out, but loosen the grip a little. The news will come when it comes. When there is news it will be visible here.
Scar Reversal and Alopecia Areata Therapies (7/1/18)
I published an article on 6/29 about Birch BioMed, a Canadian biotech company who is developing therapies to reduce scarring and treat auto-immune diseases. Head on over to the Articles homepage if you haven’t gotten a chance to read the article yet.
Follicum Completes Topical Formulation (6/27/18)
Follicum announced yesterday it has successfully completed the development of a topical formulation for FOL-005. The company had been working in parallel to develop an optimal topical version of FOL-005 while an injectable version of the peptide was being used in a clinical trial. Now that the topical formula is completed it will be trialed in a further phase 2 clinical trial which will likely begin very late 2018 or early 2019.
In addition to the news of the completed topical formula, Follicum also released alengthy summer newsletteron the same day. The newsletter includes details about the company’s work in hair growth as well as diabetes and future milestones planned.
HCell Appoints New Board Members (6/27/18)
HCell Inc.announced this week in a press release the addition of two new members to its Board of Directors. Robert P. Ryan PhD and Marlene Haffner MD PhD comprise the additions to the Board. HCell mentions inthe releasethat the respective additions will be supportive to HCell through their combined experience in orphan drug development and FDA regulatory processes.
All in all, the news is a positive sign that HCell is continuing to pursue its development of combinatorial PRP + biologic for androgenic alopecia and areata in the US.
Organ Technologies Raises Millions For Hair Tech Development (6/23/18)
It looks like Organ Technologies’ recent announcement of its hair cloning progress has attracted some investment capital. Earlier this week, Organ Technologies issued a press release announcing that they have issued new shares to three new investors in exchange for approximately 590 million yen. That’s a lot of yen. In US dollars this converts to roughly $5.3 million, still a good haul.Thepress releasementions:
“This investment is allotted to the funds for research and development of the OGT including the development for theoffer of the hair regenerative medicine.”
News was spotted on Fuji Kaurazaka’sTwitter,who has been reporting well lately.
Sangamo Therapeutics Hair Growth Research (6/18/18)
It appears that the gene-editing company Sangamo Therapeutics has an interest in hair growth technology. Sangamo is an interesting company with technology applications in gene therapy, genome editing, cell therapy, and gene regulation. Gene therapy, especially, is at the cutting-edge of medical technology innovation. The company has multiple therapies at phase 1/2 of the clinical trial process for various diseases.
Things get interesting when we discover a patent which was filed by Sangamo in May 2017 titled “Targeted Treatment of Androgenic Alopecia.” As with virtually all patents, the lengthy text of the patent is difficult to read or to create a concise summary from. An intriguing aspect of this news isSangamoworks in several technology spaces, including previously mentioned genome editing and gene therapy, either would make an advanced type of hair growth therapy we have never seen before. One caveat to mention is the company’s pipeline does not currently display any indication for alopecia, meaning the therapy is not fully developed yet, so it will be some time before trials begin. We certainly hope to hear more from Sangamo Therapeutics as soon as possible about their interesting development for hair growth technology.
Source:Fuji Maru Kagurazaka’s Hairlosscure100 blog
Official PR From Organ Technologies (6/10/18)
我也e just come across the official press release from Organ Technologies (the biotech company which is developing Tsuji’s methods) regarding their recent advancements in hair follicle cloning. It contains the important information which has already been listed on Follicle Thought, though it also includes many more details. The press release is titledOrgan Technologies and RIKEN Launch Preclinical Tests in Hair Follicle Regenerative Medicine.
Found on Fuji Maru Kagurazaja’sTwitter.
More Details on Tsuji Progress (6/5/18)
A new article from the Japanese media outletAsahi Shimbunhas surfaced with a more detailed explanation of the Tsuji/Organ Technologies progress. Here’s the key points from the latest Tsuji update:
- The latest development for the team has been creating equipment which efficiently mass produces follicle cell culturing.
- Starting in July 2018 Tsuji’s method will again be tested in the backs of mice and if all goes well clinical trials will begin in 2019.
- Clinical trials on humans in 2019 could potentially lead to commercial release in 2020, in a perfect scenario.
Sourcearticle.
New Hair Growth Pipeline Chart (6/1/18)
我希望你们都在这个星期五感觉良好。我也e created a new gannt style chart to display all of the companies from the Ultimate Guide to Hair Regeneration. In this first version I decided to keep the companies listed in the order they appear on the Ultimate Guide list. I hope you enjoy it and feel free to share this. Happy Friday.
Medipost Also Working On Clinical Therapy (5/28/18)
After the new article featuring Medipost’s hair growth cosmetic was published, Jay Lee PhD of Medipost, began chiming in on the comments section. He first shared that Medipost is currently engaging in a larger clinical trial for the CM3 product which would include higher scale Norwood’s. Then, in a following comment he revealed that Medipost is developing a potentially more advanced hair growth product as well. Here are his words:
“In parallel with MSC conditioned media as a functional cosmetics for hair loss that was introduced here, we also develop MSC therapy for the same indication as a therapeutic, not for cosmetics (though early in development stage)”
Jay also shared this regarding the difference between cosmetics and therapeutics:
“there is much limitation, for example, on the scope of API, ROA and efficacy claim for functional cosmetics. For example, you may not use stem cell itself or inject the product. For efficacy claim, “hair growth” or “hair loss prevention” is not allowed, but only ‘hair loss relief’.”
This may mean Medipost is working on an injectable that actually contains MSCs as well as a higher % conditioned media for hair growth. That sounds really exciting.
Feel Good Friday (5/25/18)
Thanks for checking the Updates page today. To make this a feel good Friday I will be posting an article about a new hair growth cosmetic today.
Setipiprant Phase 2A Trial Completing (5/22/18)
Yesterday marked the “estimated study completion date” for setipiprant’s phase 2a trial in androgenetic alopecia in males. The participants of the trial received 1000 mg of setipiprant by oral tablets twice a day over a 6 month period. I expect the results to be presented sometime in 2nd half 2018. Here’s to hoping for positive results from this trial and expediency in beginning a phase 2b or phase 3 trial for setipiprant. Clinical trial pagehere.
EHRS 2018 Presentation on Latanoprost (5/18/18)
This interesting presentation slide from researcher Yuliya Ovcharenko was on display at the European Hair Research meeting and shared on Twitter today.
Excel·lent review of Yuliya Ovcharenko on the new options for treating androgenetic alopecia.#Ehrs2018#Bologna@EHRS_trichologypic.twitter.com/dPxk4EdP46
— Dr. Ramon Grimalt (@DrRamonGrimalt)May 18, 2018
American Hair Research Society Summit (5/14/18)
AHRS is holding an event in Orlando, Florida on May 14-16. There are several interesting presentations being done on virtually all facets of hair science and medicine. Viewthe programfor more info.
Existing Drug For Osteoporosis Holds Promise For Hair (5/9/18)
And just like that, more fascinating hair-related research was published in PLOS Biology. A team of researchers lead by Dr. Nathan Hawkshaw of the University of Manchester have identified the drug ‘WAY-316606’ as a potential candidate for hair regrowth. WAY-316606 is an existing drug used to treat osteoporosis. It’s not clear at this time whether WAY-316606 is approved and on the market, or if it was partially developed to treat the bone disease.
Dr. Hawkshaw and his team were lead to test WAY-316606 for hair growth after studying the effects of cyclosporine A (CsA) on hair growth. They found that CsA reduced the expression of SFRP1 in human hair follicles. After looking for other drug candidates that had a similar effect on SFRP1, WAY-316606 was identified. The team has already tested WAY-316606 on isolated human hair follicles which were donated from hair transplant surgeries, and plans to test the drug in human clinical trials in the future. A timeline for a human clinical trial has not been set yet, Follicle Thought will update this as news is presented.
Sourcearticle.
New Understanding of Gray Hair Cause (5/8/18)
Researchers from the NIH and the University of Alabama, Birmingham have discovered a connection between the body’s innate immune regulation and hair graying. It was found that the transcritpion factor known as MITF, which plays an important role in melanocyte function, also plays a major role in hair graying. When the body’s immune system is dealing with a pathogenic infection such as bacteria or virus, molecules called interferons will send out signals to the body to take action against the pathogen. If MITF loses control of interferon response in melanocyte stem cells (due to an immune system response), hair turns gray. Essentially, immune system response may contribute to the process of hair graying according tothe study.
This immune related mechanism appears to be the same pathway that RiverTown Therapeutic’s RT1640 acts upon to reverse gray hair.
The research paper by Melissa Harris et al. was published inPLOS Biologythis month.
Histogen Receives FDA “OK” For Female Trials (5/4/18)
One of the longest running companies in the hair growth industry, Histogen, has been granted an IND from the US FDA for the use of its growth factor biologic (HSC) in female androgenic alopecia. The product will be called “HSC660” for use in women. IND stands for ‘investigational new drug’, and is a necessary permit that companies must obtain before starting clinical trials with a new medicinal substance.
Histogen founder Dr. Gail Naughton told the website Xconomy that her company is also “moving toward” a late stage clinical trial for men in Mexico. No timetables have been given about the start date for a women’s trial in any of the initial reports.
Sourcearticle.
Approved Drugs Shown to Stimulate Anagen Phase (5/4/18)
Researchers from UCLA in the lab of Jing Huang have recently shown that certain molecules which activate the cellular process known as autophagy also drive hair follicles into the anagen (growth) phase. The researchers studied different metabolite molecules and other molecules which are FDA approved and on the market as drugs. The most recognizable drugs from the study were metformin and rapamycin, one is a diabetes medicine and the other an immunosuppressant. Dr. Huang says her lab is looking to study these drugs for human hair growth soon. In my opinion, results from that study are something to look out for. Full article on the Articles page.
Concert Pharma Enrolls Phase 2a Trial for AA (4/25/18)
Yet another company has made news this week for phase 2 trial progress. Concert Pharmaceuticals announced today that they have completed enrollment for their phase 2a trial using CTP-543 in alopecia areata. CTP-543 is an oral JAK inhibitor which acts on JAK 1 and 2, it’s also known as ruxolitinib. Concert’s version of ruxolitinib has been modified by the company’s proprietary deuterium chemistry technology which the company hopes will improve its effects on AA.
The trial will be completed over the course of 6 months and Concert Pharma hopes to release data from this trial by the end of 2018. For more visit Concert’snews release.
Follicum Trial 50% Enrolled (4/23/18)
Hair growth company, Follicum of Sweden, announced today that their phase 2a trial for scalp hair growth has reached over 50% enrollment. The trial is scheduled to enroll 60 patients total who will receive injections of FOL-005, Follicum’s hair growth peptide. The study is designed to evaluate the hair growth response from different dosages of FOL-005. Patients receive injections 3 times per week for 3 months total, for the duration of the study. The study is expected to be completed in 2018. Let’s hope Follicum finds some healthy and eager volunteers to round out their study pool asap.
Full releasehere.
The Ultimate Gets Updated (4/21/18)
我也e been updating theUltimate Guide to Hair Regeneration 2018a bit over the last several weeks. There are two new companies who made the list, although you’ve most likely heard of them before, and some positions have changed. Position changes usually happen when pivotal news gets reported or progress is made by a particular company. I’ve been meaning to work on the Guide for a while now and only recently found time for it in between writing new articles and other activities.
Updated Brotzu Article (4/16/18)
New information regarding the Brotzu presentation data has been circulating the internet. I have updated the recent Brotzu article to reflect that. This new information may change the perspective of those who have been following the lotion’s developments over the past few days. Visit the main page to learn more.
Brotzu Result Photos Revealed (4/15/18)
Yesterday, result photos of the highly anticipated Brotzu lotion hit the internet. Reactions have been mixed thus far and a lot of opinions formed from just two sets of before and after photos. Here is a side-by-side comparison of the female subject at 6 months from the Brotzu presentation.
Brotzu Conference This Week (4/10/18)
For those of you who only check the Updates page, there was a new Brotzu Check-In article published yesterday. Giovanni Brotzu will be presenting data pertaining to his lotion’s use in androgenetic alopecia at an Italian hair research Congress this Saturday, April 14th. We hope to see photo results from the presentation. Check back to the Brotzu article next week for updates.
RepliCel On Point in 2018 (4/5/18)
If you haven’t already read the article, head over to the Articles section of this website and check out Replicel’s anticipated happenings for 2018. As of March, the company is still expecting data from Shiseido this year, which is a great thing.
Feel Good Friday (3/30/18)
I hope you’re all having a good Friday and feeling good out there. Two weeks ago I posted my first “Feel Good Friday” update which contained a hilarious video of Deion Sanders showing off his new hairline from a 5,000+ FUE procedure he had in 2017. That video is now unable to embed because its owner changed his account to private.Coincidentally, a week after I shared that video I noticed that Deion posted a new video to his own Instagram account of him going back to his clinic for another FUE hair transplant to “comeplete his comeback”, in his own words.
This video is equally hilarious. Once again, there is inspiration here. Deion had a 5,000+ FUE last year and received good results from it. But he’s not done yet, he has the resources to have another procedure and get as close as he can to the result that he really wants, so he did. Surely, he’s just about maxing-out his donor area now. Once again, congratulations to Deion for pursuing his hair growth goals. Enjoy the video and have a big laugh. OOOOeeee.
Gene Therapy Studied for Hair Growth (3/26/18)
Newarticlehas been published on CAR-T therapy being reportedly studied to treat hair loss. CAR-T therapy is a new innovative procedure utilizing a patient’s own immune cells known as T cells. The only source of this information so far is a Turkish newspaper, however the reports appear very likely and credible.
Featured Products Section & More (3/25/18)
我也e recently added a new section of the website which will be dedicated to worthwhile products which can be used in an everyday hair growth regimen. The first product to make the list is the Teslabrush. I’ve appreciated the candid and practical commentary from Teslabrush inventor Bernhard Rudert on what his product is capable of. I’m also in favor of the fact that the Teslabrush does not replace other treatments one may be utilizing, but instead, Bernhard has said it works well with other hair growth treatments and has been shown to enhance them through combination. There is more information and commentary from Bernhard on theFeatured Productspage. I’d like to hear from readers who try this product, please feel free to share your reviews on how it’s working for you in the HairCell: New Websitearticlewhere the Teslabrush was first mentioned.
On Monday 3/26 I plan to release a new article on a major pharmaceutical company who appears to be delving into hair growth research. Things seem to be early for them, but it’s an interesting one. I hope you enjoy it
New BBC Documentary about Hair Loss (3/21/18 – Updated)
BBC Newsbeat has released a documentary yesterday titled “Too Young To Go Bald.” The program gives a candid look into the lives of several young adults dealing with various forms of alopecia. Chidera Eggerue, a blogger dealing with traction alopecia, meets up with a female British rapper who previously underwent a hair transplant for her traction alopecia. Also in the documentary, vlogger Perry O’Bree who is dealing with androgenic alopecia shares about his own experiences.
Unfortunately, as of now the video is only available onBCC Newsbeatfor people living in the UK. I haven’t been able to watch it yet but am searching for a solution for those of us abroad to view the episode. One of the personalities featured in the film, Perry O’Bree, has created an interestingYoutube Videopromoting the message that #HairLossHappens and that those who experience it are not alone. I find it to be a courageous and uplifting message. The topic often is often overlooked and understated, and the truth is that hair loss ismuchmore of an important issue than how it is portrayed in society. Kudos to Perry.
Update: A reader “Welsh Dragon” from the UK area has commented below with a more thorough synopsis of the documentary. Also, this reader has left an inspiring quote in the comments section and I thought it was so admirable I’d like to share it here.
“No probs. If you come across any other documentaries in the UK that you cannot view in the States feel free to ask me to watch it and feedback. I think sites like yours give people an incentive to keep looking forward with some optimism. Without doubt a feasible hair loss solution isn’t far away. I think it will most likely be a next gen hair transplant through hair cloning. But what ever it might be, as you said in a previous comment there have never been as many players in the hair loss industry. There will be a few false starts, but one, quickly followed by others will come through. It’s just the wait ? But you never know with Shiseido, Brotzu and Haircell releasing data this year it could be sooner than we think ? Kind regards” – Welsh Dragon
Feel Good Friday (3/16/18)
Happy Friday from Follicle Thought and I hope you are enjoying March Madness basketball. That being said, please also enjoy this video of Deion Sanders, NFL Hall of Famer who recently had a 5,000+ FUE hair transplant, showing off his new grown-in hairline. Video was posted by his barber. With all the technical details and the day-in, day-out watch for new hair growth technology it is important we take a moment to enjoy the lighter side of things. Sit back and “get a side shot of that thing.” ?
(The Instagram account which the video was posted on has changed to private and the video can no longer be shared)
Multiple Alopecia Trials for Aclaris (3/16/18)
Aclaris Therapeutics, the company who acquired the rights from Angela Christiano to use JAK inhibitors in alopecia disorders, is currently involved in a wide range of alopecia trials. The company has multiple ongoing trials for alopecia areata, including a trial for eyebrow regrowth, and also a new trial planned for AGA or androgenic alopecia. Full articlehere.
Update from Yokohama National University (3/13/18)
Head over to the main Articles page to read an update from head researcher Junji Fukuda PhD of Yokohama National University. This is the research that spurred the “french fry hair cure” craze of recent news headlines. I asked Junji in a Q&A if he has received any interest from bigger companies to commercialize his technology. Find his answer and more insight to what lead him to hair follicle research in the full article.
Carboxytherapy Found Effective For Alopecia (3/10/18)
A study led by Dr. Noha Doghaim of Tanta University in Egypt showed that carboxytherapy may be a promising treatment option for both alopecia areata and androgenic alopecia. The study comprised 80 subjects who were treated over the period of three months with either placebo or carboxytherapy.Both groups found favorable results from the carboxytherapy, however during a follow-up examination the improvements in androgenic alopecia subjects had decreased over time. The researchers noted that continual treatments would be necessary to maintain and bolster the benefits for AGA.
Carboxytherapyis a non-surgical cosmetic medicine treatment which employs injections to infuse gaseous carbon dioxide below the skin into the subcutaneous tissue through a needle.
Sourcehere.
Aclaris Issued New Hair Patent (3/9/18)
Aclaris Therapeutics has announced on March 9, 2018 that the US Patent and Trademark Office has issued their company U.S. Patent No. 9,895,301, for methods related to the use and administration of a certain janus kinase (JAK) inhibitor for treating hair loss disorders. This patent covers the treatment of both alopecia areata as well as androgenic alopecia.
Dr. Neal Walker, CEO of Aclaris had this to say about Aclaris’ new patent:
“We are extremely pleased with the continued development of the patent portfolio we exclusively licensed from Columbia. This new issuance continues to expand the breadth and depth of our JAK inhibitor intellectual property portfolio covering methods of use for certain JAK inhibitors for the treatment of hair loss disorders. The issuance of this patent is another step in the development of a robust patent portfolio relating to JAK inhibition and hair loss,”
Full press releasehere.
Phase 2a Trial Launched For Follicum (3/7/18)
It’s official. We have another company engaged in an active phase 2a trial for androgenic alopecia. Follicum has announced this morning that the first patient from their phase 2a scalp study has been treated. Here’s the headlines from Follicum’s latest press release:
Lund, Sweden, March 7, 2018: Follicum AB (“Follicum” or “the company”) today announced that the first patient has been treated in the Phase IIa clinical trial in Germany with its lead candidate FOL-005 on 60 patients with hair loss. The study is conducted at Clinical Research Center for Hair and Skin Science (“CRC”) in Berlin and bioskin, Hamburg, Germany. The global market for pharmaceutical hair loss products for both men and women is estimated to be worth $3 billion. The available drug products have unwanted side-effects that limit their use.
Full press releasehere.
HairClone 2018 Update (3/2/18)
HairClone of the UK has recently released their third newsletter with company updates. Among the significant updates in the newsletter is the mentioning of a crowdfunding campaign video which is ‘ready to go.’Articlessection has full details from the newsletter.
Cynata Stem Cells for Hair (3/2/18)
If you haven’t done so already, please check out of the Articles section to read more about a company working in the stem cell industry who has an interest in creating a therapy for hair regeneration. Cynata Therapeutics has a very interesting platform capable of creating an unlimited supply of stem cells for clinical use.
Clear Clinic Website Up (2/21/18)
The English version of the Clear Clinic is up and functional. On the site I noticed an abundance of Before & After photos. Link is in the Clear Clinic article on main page.
Brotzu Speaks on Final Countdown (2/19/18)
The Brotzu lotion has been back in Italian headlines over the past week. It’s not clear what has prompted this wave of Brotzu news, but we know there has been significant interest surrounding the lotion since it first spread across the international hair-ternet. Recently, enthusiasts have also gone as far to start an online petition advocating for Fidia Pharma and Brotzu to release the heralded lotion.
In this article Italianarticle(requires translator), Dr. Giovanni Brotzu offers some new insight as to what’s going on with the lotion. Translated via google he says:
“The road to the marketing of the product has not yet been completed….we are continuing to work to stabilize the preparation and make it suitable to public distribution.”
“I have the email clogged by those who ask me news about the lotion and the phone does not stop ringing, but there is no mystery we are at the end and soon we will understand if the treatment will have the reliability requirements to enter business.”
“The lotion works (it) has been tested on sixty volunteers and has confirmed its effectiveness.”
It sounds as though a formula is now being put to the test to see if it will remain stable for a suitable length of time and be effective for us. The news is encouraging and helps alleviate the ongoing uncertainty surrounding the product. Let’s hope that some top notch chemists have been able to work some magic for Fidia and the rest of us.
Found onReddit.
More Fun More Follicum (2/7/18)
Follicum’s new phase 2a study for the scalp has been approved by the German Medicines Agency. The study is expected to be completed in 2018 and more importantly, results from the study are expected to be announced in 2018 as well. Press releasehere.
New Follicum Interview (2/6/18)
New Youtube video interview with Follicum CEO Jan Alenfallhere.
Yokohama University Hair Follicle Cloning (2/5/18)
I must have had about 5 readers email me today about the fascinating research coming out of Yokohama National University in Japan. Professor Junji Fukuda lead the efforts to successfully prepare “hair follicle germs” at large scale simultaneously. Essentially, the researchers prepared a cellular formulation with the right culturing materials to promote successful growth and development of tiny “hair follicle starter kits.” Source articlehere.
Researchers from A*STAR Study Follicle Metabolism (1/30/18)
Some interesting research has come out of Singapore’s A*STAR institute led by Thomas Dawson. Dawson and his team believes they have identified a correlation between age-related hair loss and the slowing of metabolism. Different than androgenic alopecia this condition is called ‘chronogenetic alopecia.’ For more please review thesource article.
Replicel Announces New Asian Partnership (1/25/18)
Replicel has secured a key investment and partnership from the YOFOTO Health Industry Co. of China. YOFOTO will be leading clinical trials for Replicel’s tendon repair (RCT-01) and skin rejuvenation (RCS-01) therapies in China. Shiseido maintains the license to use the hair growth therapy (RCH-01) throughout all of Asia. But it’s good news all around as YOFOTO will provide money for Replicel’s continued development. New partnerships are agood signfor Replicel. Next stop is the that Shiseido data, I’m hoping for the best.
Fast Track Granted To Alopecia Areata Therapy (1/24/18)
Originally spotted this onHairLossCure100’stwitter page. Concert Pharmaceutical’s therapy for alopecia areata, CTP-543, has been granted fast track designation from the FDA. CTP-543 is an oral JAK inhibitor (ruxolitinib). From what I’ve read,fast trackdesignation encourages early and frequent communications between the FDA and the company during the development process to ensure issues and questions are resolved quickly.
HCell New Company (1/15/18)
Literally jumping right out of the woodwork, the company“HCell” has announced they have been granted an orphan designation from the US FDA for their novel treatment of pediatric alopecia areata. The treatment itself it described as a “topical Injection by regenerating hair through a proprietary blend of commercially procured biologic and autologous tissue.” The company also mentions having a treatment for androgenic alopecia in the works as well. More info to come soon. News releasehere.
Tesla Brush Photos Added (1/5/18)
Before and After photos, as well as commentary from the inventor of theTesla Brushhave been added to the newHairCell文章从主页面。
Calling All Investors (1/4/18)
I am seeking angel investors or venture capitalists that are looking for promising hair growth technology to invest in. Please get in touch with me via the世界杯2022入围名单 .
Hair-Bearing Skin Created In Lab (1/2/18)
Scientists from the Indiana University School of Medicine have for the first time created skin with hair follicles using mice stem cells. Research was led by Professor Karl Koehler. The team was able to grow both the epidermis and dermis layers of skin to create a realistic skin model. An interesting quote from Professor Koehler: “It looks like a little ball of pocket lint that floats around in the culture medium. The skin develops as a spherical cyst and then the hair follicles grow outward in all directions – like dandelion seeds.”
Initially the research was aimed at treating deafness by growing tiny versions of the inner ear in vitro. When the team observed that skin cells were forming as well, they attempted (successfully) to get the skin to grow hair follicles. Full articlehere.
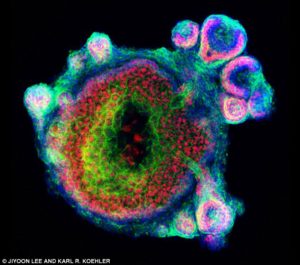
Happy New Year (12/31/17)
A Happy New Year! to all of my readers. I wanted to check in and let everyone know I will not be doing a 2017 recap post which has been a tradition over the past 2 years. I will, however, be doing a 2018 look ahead post very soon. Thank you all for supporting Follicle Thought and I wish you a happy, healthy, and joyful 2018.
HairCell New Website (12/19/17)
I took a look at the new HairCell website and highlighted all of the important factors in my latest feature article. Click the Articles link to check it out.
Japan Hair Blog Article and Efforts for Solidarity (12/29/17)
A very kind and talented hair blogger from Japan,Fuji Maru Kagurazaka, recently contacted me and suggested that the blogs aimed at promoting hair growth treatments/cures for hair loss should unite their efforts and cooperate. While I acknowledge that the websites who are focused on this material are each unique and have their own (and sometimes differing) perspectives, I do believe that civility and solidarity are certainly desirable qualities for this sector. Fuji wrote up thisnice articleabout my website, check it out (make sure translate is on if you are not fluent in Japanese). And, I encourage you to read his other articles. Fuji has a sincere and highly detailed approach to hair growth blogging. His personality is highly welcomed and refreshing to the online hair community.
Rophe Pharma Used on Eyebrows (12/23/17)
Rophe Pharma has issued a press release detailing the use of Rohpe’s topical hair growth drug on eyebrows. The news ended up on Yahoo. Check the Articles section on Follicle Thought for the full scoop.
Follicum Season’s Greetings (12/21/17)
新的文章已经与公司发布在网站上mation from Follicum’s CBO Gunnar Gardemyr. Details include three topical formulations Follicum is working on for its hair growth drug FOL-005. Check the Articles page on this site.
Kerastem Reports Successful Phase 2 (12/09/17)
Kerastem, a company developing an autologous fat-derived stem cell therapy for hair growth, has reported positive data from their phase 2 trial. The results have come from a 6 month clinical trial involving 70 patients. In this study, the patients received a one-time injection of fat-derived stem cells, and purified fat, into their scalp. Kerastem reports an average increase of 29 hairs per cm2from the treatment, or an increase of 17% from baseline. Thepress releasedoes mention that the treatment “successfully stimulates hair growth in people withearly stagehair loss”, so that is something to take into consideration when evaluating the results. For more info visit Kerastem’swebsite.
Replicel CEO Expects Clinical Results In 2018 (11/24/17)
Lee Buckler, CEO of Replicel, stated in an interview this week that he expects Shiseido to release clinical trial results in 2018. This is great news that everything is still on track for the anticipated 2018 release of Replicel’s RCH-01 technology in Japan. Lee mentioned“It’s entirely up to Shiseido what they do in regards to this product. There’s certainly a possibility that they could decide if the data is positive, to launch the product in Japan…”.Yes, it seems likely that if the data is positive, Shiseido would go to market with one of the biggest technological breakthroughs of the century. Full interviewhere.
South Korean Researchers Developing Hair Growth Protein (11/21/17)
Researchers from South Korea have identified a new peptide called PTD-DBM which exhibits wound healing and hair regeneration effects in preclinical studies. The research is beingled by Professor Kang-Yell Choi of Yonsei University. Choi’s team identified the peptide PTD-DBM which targets a protein called CXXC5. The interaction of these two proteins leads to stimulation of the Wnt pathway, which then initiates hair follicle neogenesis. Choi hopes to develop this peptide further into a potential hair growth drug candidate. Aresearch paperabout these findings was put out by the team earlier this year. Source article about this developmenthere.
SCM Lifescience Working On Hair Growth (11/16/17)
We have yet another biotech research company working on a next-gen hair growth cosmetic product. Their webpage mentions the use of “stem cell culture solution-derived proteins” for hair growth cosmetic products. An imminent release of the product seems unlikely, however it is nice to know how many companies really do want us to have a new product to improve our lives. Websitehere.
New Article with Quark Pharma WCHR2017 Presentation (11/14/17)
Check the Articles homepage to view an interesting presentation from Quark Pharmaceuticals pertaining to their new siRNA therapy for AGA and chemotherapy induced hair loss.
Please Support Follicle Thought (11/8/17)
I’d like to take this time to formally invite everyone who has found this website to be a useful resource in their daily lives, to make a contribution to my donation page on Patreon.
I know that Follicle Thought has been very useful and inspiring to companies, doctors, and people interested in hair restoration alike. A one-time pledge to my donation page would make a difference in my life and be very appreciated. To make a one-time donation simply click “Become a Patron” on the right sidebar of my Patreonpage.Then you can adjust the amount of how much you’d like to contribute; Patreon will call this a “monthly contribution”, however you can cancel your pledge after one month, hence making it a one-time pledge. ? I am thankful to all of you who take a moment to visit the page and make a contribution, it means a lot, especially for the hours upon hours I have put into the site to make the best information available to you all.
Stem Cell Company Working On Cosmetic Hair Growth Product (11/7/17)
A little something for you all to nibble on. I’ve recently discovered a stem cell therapeutic company who has an interest in putting out cosmetic hair growth products. The company, Stemedica, is working on treating a wide range of diseases through clinical stem cell therapies. I counted 6 clinical trials in progress on their pipeline page. Perhaps more interesting for this audience, the company is also developing skin and hair growth cosmetic products from their core stem cell technology. Stemedica has a specific subsidiary in place to roll out dermatology cosmetics called StemCutis. Their website mentions the use of stem cell-derived growth factors to to be used in the products. It’s not clear what stage of development the hair growth product is at, but it’s good to have it listed inStemedica’s sights.
World Congress For Hair Research 2017 (10/31/17)
The annual World Congress for Hair Research kicks off today in Kyoto, Japan. The Congress will go on until Friday November 3, 2017. The purpose of the event is to share presentations about different lines of research and product development regarding alopecia areata, stem cell biology, androgenic alopecia, cosmetic care, wigs, hair transplants, and more. Basically, all things hair industry. At these conferences a few companies typically will announce some news about their developments and progress. I’ll update any news I receive regarding announcements made at the Congress.WCHR2017 website.
JW Pharma, UPenn Collaborate On Hair Growth Therapy (10/30/17)
JW Pharma, a Korean biotech company that specializes in the Wnt pathway, has entered a cooperative agreement with the University of Pennsylvania to development a compound called CWL08006 for hair regeneration purposes. JW Pharma has a pool of Wnt targeting therapies, and apparently CWL08006 makes a great candidate for hair growth. Of course, Dr. George Cotsarelis of UPenn is involved, and the two teams plan to begin preclinical testing by 2019 (I’m a little surprised at that timeline for preclinical). One of the more interesting facets of thereport, JW intends “to accelerate its commercialization by launching tests on the human body for cosmetics that utilizes the drug’s mechanism, next year.” If the candidate works well in the cosmetic tests, a version of it could be made available much sooner than the clinical drug pathway, but for now it’s not possible to speculate a time frame for that yet. Good news all in all.
Deion Celebrates New Hair (10/29/17)
NFL Hall of Fame player Deion Sanders has recently undergone a hair transplant. He has not been shy about discussing it publicly and over the last several weeks has actually continued to put out a slew of hilarious and outrageouspostspertaining to his renewed follicles. For his first announcement to social media, he posted this jubilant and priceless video to his Instagram:
There’s no better feeling than getting your hair back– just ask@DeionSanders! Check out his recent Instagram post:https://t.co/Y7M504PCfPpic.twitter.com/c90dncVLhF
— RESTORE Hair (@restore)September 27, 2017
我也e been looking forward to sharing this with you all. It makes me laugh everytime I watch it. But really, look at how happy he really is and how much joy he feels from restoring his hair. The man is an NFL Hall of Famer, also played MLB, and made millions of dollars throughout his career; and he looks like a kid getting free ice cream on a Friday afternoon because his hair grafts are beginning to sprout. I find it inspiring, to be honest. Keep dreaming, keep believing. We’re all looking forward to having our own “I got some hair!” moment.
New Company Working On AGA Drug (10/27/17)
A company not previously discussed in online news,SWITCH BIOTECH, has sights on developing a drug for androgenic alopecia. As you will see, the company is still in the very early stages of developing a therapy for AGA, however they are a knowledgeable dermatology-focused company and have a unique method of drug development. Here’s a quote from their website about working on AGA:
“SWITCHBIOTECH intends to develop a drug that either prolongs the hair cycle, stops the miniaturization of the hair follicle, or blocks the signals from the dermal papilla that inhibit hair growth.”


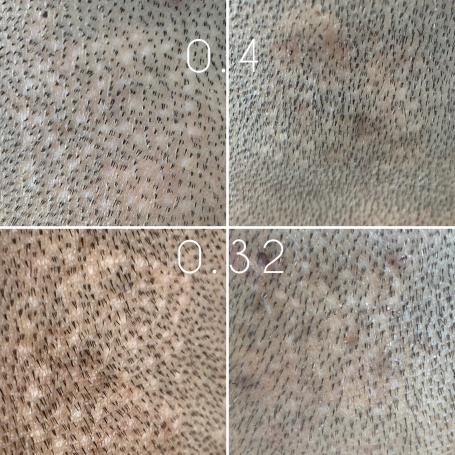
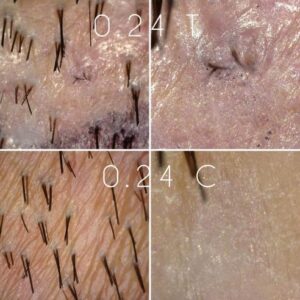
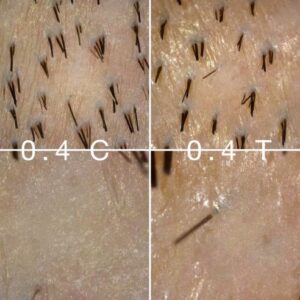



Hi,
缺少一个完整的头发移植,我们大多数人都勒ft to cope daily with the limited benefit of drugs and the profound loss of personal and sexual confidence. Loss of ones hair can push someone into a real state of depression and anxiety, which are real changes to the balance of our chemical biology. So my coping mechanism for my hair loss is to boost my mood with aerobic exercise. Its benefits on clinical depression have been documented and are real. Running lifts my mood and puts me in a feeling of control. It is quite the magic pill and offsets the doom I feel of being out of control over my hair loss. Ask yourself why are there men who feel perfectly confident despite being bald. Maybe they too have found other ways to boost their physical and emotional life with exercise or other modalities. Either way you have to take action and don’t just sit and wait for some drug or lotion to change your life. I am doubtful. Ask yourself if you had your hair back would your sense of inadequacy really change or would it just be short-lived vanity. Take losing your hair as a way to work on you as a person and lift yourself because if not your hair one day you might lose something that cannot grow back or heal. I am moved by seeing disabled people every day live their lives as full as they can and without fear. Do the same. No one cares that we are bald and if they do, they are just vain and petty. Good luck everyone.
Very nice response Joe. A good perspective.
I’m curious, since you said you are doubtful about new treatments, what continuously brings you to this site? A shimmer of hope?
Kindest regards
Hope dies last.
Best wishes to you
I think the answer is shave your head and the worrying would be over. If not for a FUT scar that is what I would be doing. I am making an appt to see about a scar revision and if it goes well enough, I will chop it down to a stubble. I am in a losing battle and don’t see anything coming out in next 2 years that will make a huge difference on growth – maybe on retention, but I have lost too much already. Would love if I am wrong. But, the overwhelming majority of people you read seem to have found some peace once they save it off.
I’m very thankfull for This Site. It was my “daily bread” to see If there is a new Update or a new Treatment on the run. I followed it with high hopes and a Heart full of bitterness, because i loved my full, long Hair. It was the only thing i liked on my appearance. With my Hair i lost a Lot self confidence, happyness and quality of life.
To Accept it, Well, i really tried it, but nothing helped me there. I can Not acept it and i don’t know why.
However in the other hand i lost my Hope in a new Treatment.
Some of them seems to be to expensive (it is hard enough in my young years to spend more than 1000 Euros) or not effective Like FOL-004, which seems to ne nothing more than Finasterid + microneedling.
yeah joe i used to run alot and i’d get a runner’s high i did cross country in high school and when i was in collage a couple of years ago i continued to run but i stopped now i just go on long walks and sometimes play tennis to get my mind off of hair and try and take a mental break.
Why is samumed delayed in relasing the result of SM04554/dalosirvat..do you have any influence in asking them directly will the resutl will be released or not?
We will have to wait for when Samumed is ready to release the data Kattappa. Delays are not always the best indicators, but we will have to wait to find out the truth.
there’s already a cure for hair loss. DHT blocking shampoo twice a day. Minoxidil nightly. Propecia daily or natural DHT blocking pill. Derma roll once a week. I have yet to see anyone follow this regime and not completely regrow their hair.
没有t sure if this could really regrow dense hair for any bald area (even a small circumference) but it does sound like a dedicated and thoughtful regimen.
That sounds great, but in reality it’s not the case. Shampoos have little effect. Propecia loses effectiveness over time, and you will continue losing your hair. And most men do NOT regrow all their lost hair just some of it.
Never lose hope. What humans have accomplished thus far is astounding and the research and development for hair loss should be taken as inspiring. If we lose hope, what do we have? We should all help each other raise awareness for these causes that are trying, as a lot of people are affected physically by hair loss but more importantly mentally. Give encouraging words to these companies, It goes a long way.
我也e noticed a few people giving advice on how to overcome the experience of living with hair loss. These are great sentiments which create camaraderie and you’re all welcome to share more.
I’d like to re-state the true intention of my update was to ask people what they can do to help contribute to a new treatment becoming available, and thus, feel empowered in the situation. Rather than just leave the task for “someone else” to take care of.
我仍然相信任何人来的主要原因is site is that they have hope beyond hope that the reality of hair growth options will change and improve.
Hi my friend! I would to know why in your ultimate update of thE PIpelinE It isnt be yet the Tsuji Solution ???
Hi Alvaro, at the time I updated the chart it seemed that Tsuji is quite far from funding a clinical trial. I still tend to believe that is the case, but, I have read the recent article which is creating some confusion. Will try to get a clearer answer on this.
Thank you very much for.the answer my friend and my best wishes for all of you
New Alleged Brotzu/Trinov Photos
Hey!
So I saw above you mention a company trialing Demodex hair product and I’d defo be up for using it!
Will the link be posted via email?
The link will be shared in the paragraph of update on Monday. Thanks for your interest.
FT,
关于BBC之前您提供了一个更新documentary.
I noticed they have produced another short piece following a young man in his journey to get a hair transplant but also touches on the underlying issues associated with people wanting to change their appearance.
It can be viewed on Facebook:
https://www.youtube.com/watch?v=JKdorI9o2rg
Did anyone hear of new trials for a refined cyclosporin, starting in Italy and lead by English scientists
@admin
Regarding the RCH news, is your source more reliable than Lee Buckler since he seems to know more about RCH than Lee buckler himself. Recently, Lee Buckler tweeted “We anticipate seeing data from the RCH-01 study in Japan sometime before year-end but the real answer is whenever the investigators/hospitals in charge of the trial (not us) decide to release the data once it ready”
Where did Lee say that?
https://twitter.com/RepliCel/status/1050079652507598848
Thanks for the link, I believe my source is accurate.
He tweeted that 2 months ago i think
@admin
Has the trial officially finished ? If yes, any hints regarding the results ?
I don’t have a definite answer on completion, but I think it is still ongoing currently.
@admin:
If the trial has not been concluded, it would be quite puzzling since 2 years should have been ended (since 26 July 2016). Nvm, thanks for updating.
Processes are subject to change Kira. Thanks for your continued support of Follicle Thought.
I will edit the update, yet I’d be surprised if the site is not legitimate at this point, even if it is not official from Fidia. Prudency works though, thanks for the comment.
再保险10/17/18后我真的很喜欢你的回应to the request made by one of your readers to email companies asking for information. So I decided to do what you suggested and sent emails to Organ Technologies, Rivertown and Follicum (no particular reason) basically thanking them for their work and wishing them success with their products. Interestingly I received a very nice response from Organ Technologies which really surprised me. Correct me if I’m wrong but I think the sentiment is to be thankful and encouraging to what is being done appose to being negative that we have yet to get what we all want.
Welsh Dragon, you’ve been such a welcomed contributor to Follicle Thought, Thank You.
You got it perfectly there and I am inspired by the fact you took initiative to reach out to those companies and make a difference. I hope others will follow suit. Cheers to you
谢谢你!你说的真好。
I also reached out to Histogen and Follicum a few weeks ago as well thanking them for all their hard work in bringing a safe and effective treatment to people all over the globe with hairloss issues and expressed how much we all value these companies. I held back from asking about market release as you had suggested. I received a very nice reply from Histogen.
So on that note, Thank you for this site and the service you provide, it really does instill hope that very soon there will be several safe and viable treatments options for us all!
Trinov is already in pre-sale at pharmacies
https://www.farmaciasantantonio.net/trattamenti-anticaduta/1564-trinov-lozione-anticaduta-donna-30-ml.html
Hi Everyone, Just checking to see if anyone has heard when we in the USA can order Trinova? I look at the order page from your link and they are never in english and its very frustrating waiting. Hope all is looking up.
Craig, just off the bat I would recommend you use a browser than translates web pages. It’s a very common feature among web browsers.
Hi Admin I use Google and it lets me translate some web pages but I have not found that option trying to do so on all of the Trinov information sites when looking for options to order Trinov. Please let us know anything when you find out .As always thanks for all your help and information you provide to us.
Will it reverse gray hair
Happy new year FT,
The Follicum update is a great way to start the year in my opinion. If Bradford Uni can provide Follicum with a better understanding of their potential product it will probably result in more accurate and successful trials and a quicker root to market. Cheers for this update.
Happy New Year!
In reviewing the pipeline I thought Histogen was in phase 3 ?
Also, has anyone bought and started using Trinov yet?
Thanks for sharing information with us. This is informative article.
New update from follicum.
Ugh! When is the sheisheido announcement going to happen! The suspense is killing me. Q1 this year right?
Admin,
Thank you for the updated pipeline.
Are you able to confirm if setripirant and breezula are intended for females ? I know histogen is testing their product on females, any idea if the other treatments will be indicated for females as well as males?
Yes, setipiprant is for sure, I am not certain about breezula.
Allergan completed its phase 2 setipiprant, posting results to clinical trials. gov on April 5th, 2019. Does anyone know when will will see these resutls?https://clinicaltrials.gov/ct2/show/study/NCT02781311
Click on the Study Results tab on the page you shared. Scroll down to Outcome Measures and you will see the data. Spoiler alert. Unfortunately at the dose given in this study, setipiprant showed no significant difference compared to placebo in objective and subjective measures of hair growth.
Admin, are your predictions for Shiseido / replicel and Tsuji still current in late June of 2019? Ik phase II for shiseido will be coming out soon but I’d just like to know if you’re still sticking with your predictions. Thx
管理你知道研究者意味着当they said that carboxytherapy is more powerful with “adjuvants”? Are they talking about Rogaine? Because their paper don’t mention anything :/
Thanks
I would imagine that is what they are referring to.
Admin, I must say that I strongly disagree with your decision to cancel the presale of Celino’s NGF 574h. While I do admire your integrity, I personally would rather have the option to exercise my “right to risk”. I VERY, VERY MUCH would like you to provide access to this treatment. Ever since you posted about the presale, I’ve been checking the site for updates several times a day, every day. I’m nothing less than gutted by this.
You’re clearly a principled person and are to be commended, but I implore you to reconsider.
Corwin, I really appreciate your support of this site first and foremost. Secondly, appreciate the kind words about my decision. It’s funny because I deal with a double-edged sword on this subject. Some people like you want to give it a try, and on the other hand there is a potential for great criticism if it doesn’t pan out. Not everyone shares your sentiment. I’m sorry but it’s the only decision I have at the moment.
Save your money mate, for at least the next ten years.
I understand how you feel Benji, but it’s definitely not going to be ten years. Each of these iterations keeps getting better. There’s even more than one cosmetics in development right now that are being developed by top level labs. One of them has got to hit the mark.
I had high hopes for KenGro.
Let’s hope. Plenty of smart, driven and capitalistic forces are at work.
I was surprised to see them in this spot myself, but here they are. Follica is set to begin a phase 3 clinical trial in 2017. Or, maybe they already have begun this trial. Follica is kind of like the “Carmen Sandiego” of hair growth companies so no one really knows except for them. We know the phase 3 trial will happen in 2017. Pending results, this trial can lead to FDA approval for Follica. If all goes well Follica is aiming to put their micro wounding + compounds treatment for hair growth on the market in 2018. The treatment is now being titled as “RAIN” according to PureTech’s pipeline page. ..
…..what happened to the rain????….. And what happened to rt 1640
Kalvin, RAIN has turned into FOL-004 which has been talked about quite a lot on in recent articles and Updates. Please see recent Follica articles by searching “Follica” in this site. In 2017 Follica did claim they were preparing for a pivotal clinical trial, but unfortunately they have faced many delays which is quite common in this industry. A few years later here they are and finally getting closer to that pivotal trial.
RT1640 is still doing their best to raise funds for a phase 2 trial, they’ve spoken to many larger companies along the way but are still seeking a completed deal. I hope to share an update with RT1640 by this Spring.
只有今天我发现你有这个更新页面which is great.. consider moving it to main page as a monthly post that summarizes all events that happened that month.
Hi Me, thanks! The Updates page can’t be moved to the main page because it is an article which continually gets updated. But, hopefully many people will notice it. I’ve shared it many times before.
I hope there is a really effective treatment coming soon,No longer a trick,Because we know we need it
Hello Admin,
I see many advertisements of dht blocking shampoo. Does it really works?
Also see advertisement of biotin shampoo for hair growth. Is it worth to buy?
嗨Devit, DHT阻塞洗发水洗头specially formulated to contain the best natural sources of anti-DHT ingredients. It does not work like Finasteride naturally, but it is a way to get quality DHT blocking herbs into your scalp and follicles once a day or so. It’s a quality shampoo. I am not familiar with the biotin shampoo you’re mentioning because that is coming from Google Ads which are random.
Thanks for the reply. I am seeing more ads of this:https://www.amazon.in/Biotin-Shampoo-Hair-Growth-Thickening/dp/B079RDR8ZD/ref=mp_s_a_1_11?dchild=1&keywords=dht+blocker+shampoo+for+men&qid=1586832478&sr=8-11
This is biotin shampoo + having biotin
My hair falls during hair wash. So wanted to check if this really works.
“Additionally, Histogen officially became a publicly traded company last week and is now trading under the name (HSTO) on Nasdaq”
I think that can’t be right? if I look at price charts of HSTO, I see that that company has been publicly traded ever since 2013.
This article will explain GreenMonkey//www.tokobukuku.com/histogen-merges-with-conatus-pharmaceuticals/
Hello Admin, if Histogen 1b/2a trail is successful then can they go for phase 3 trail? Or phase 2b would be mandate?
I believe they will need to do a phase 2b Devit.
you may have seen this:
https://news.ncsu.edu/2020/07/microrna-for-hair-regrowth/
Hi admin, how much time will it take the precautions after the treatment, because i want to take the hair transplant.
Follicle thought have you seen this? Maybe this new drug AI drug discovery can find this same drug mechanism but not the same drug with the nasty side effects as this one because it was banned.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1498062/
Very cool, I’ve never seen this before but it’s definitely possible that AI could find a similar drug with less side effects. I’m really looking forward to hearing about the mechanism of action from Insilico’s drug.
Has anyone gave Ultrax Labs Hair Plush a try? I just saw on Yahoo news today and it has really good review on Amazon. Someone please give feedback here.
I don’t know the product Brian, but it’s highly unlikely that this product is something that has just come out of the blue and actually works well.
Hope everything goes well for Histogen and company could start phase 2b /3 trail in next year.
Admin any update on Triple hair? They were doing phase 3 trail but no news since long.
They’ve not started the phase 3 trial yet, Devit. Look for that in 2021.
Got it thank you
Great news! And that was quick I like that speed.
Follicle thought I remember now and I found where I saw “stemson therapeutics blood draw for hair cloning” I found it on this website on July 5th or 6th 2019 you posted about stemson therapeutics you said this “What makes all of this somewhat irrelevant is the fact that Stemson only needs to do a blood draw to receive all of the cells it needs from a patient! Allogeneic or not, it would be a laugh, as they say in London. Human blood cells would be converted into iPSCs which would then be converted into human dermal papilla and epithelial cells”. I don’t understand if you’re getting the cells from a blood draw it wouldn’t be a cure because you have to get the cells from the dht resistent zone you have to get the cells from the hairs on the back of your head.
Yes, we know they’re using iPSCs from patients, but if they are finding a way to create DHT resistant follicles or not, we don’t know yet. I’m sure that concept is on their radar.
follicle thought that would be really cool if they find a way to create dht resitant follicles.
I’m sick of waiting for applied biology to update us with information in regards to their minoxidil enhancing shampoo. Having read the paper they wrote in wileyonline, it looks like it’s either tretinoin compounded into a shampoo, or is no better than that, which my doctor can prescribe already. I’m going to try it and get back to you all.
Has anyone heard of this product called Provillus? This website is providing a full money back guarantee on a 90 days trial at a 50%Discount…I want to get this for my wife…She is getting into depression so I want to make sure it works…Have any of you tried Provillus before?
Good! now we can finally see if this works on a human.
I have order AAPE which is a topical stem cell for hair loss you can order it fromhttp://www.hairstemstore.com
750€ for 6 months ? No thanks..
Hopefully Melissa Harris continues work with rt1640 as it has proven to regrow hair and reverse grey hair in human and mice studies. Surely it’s too good for it to be stopped completely. I had high hopes as I suffer from both.
How about Viola Verecunda? Plant-based substance with claims that it is as effective as minoxidil. Just read that Tony moly is producing it for launch in 2021.
Thanks for sharing, I wasn’t familiar with Tony Moly and almost thought this was a joke post. Can you share the link to the product launch info?
It’s sad to hear Rivertown stopped their research from lack of investors I was hoping we were close to a reversal of greys. But glad to hear Krox20 is still going even though we haven’t heard a peep from them for 3 years. Does Dr. Lu le have some form of social media or website where we can see updates on any progress? Thanks!
Hey FT, when do you think the pig trial for stemson will last? Like a year? Because that’s how long it takes for a hair transplant hair to fully come in.
Woofy who knows? dont ask stupid questions
It’s just a question. Don’t be rude al budny
Just a guess, the “main” portion of the trial may be about 6-8 months to see how the hair comes in and make sure nothing off target occurs, then they likely take a final observation around 12 months.
Ok thanks FT.
Admin maybe pig trials take several years, who knows…
scientists at Columbia university can do a human trial doing this-https://www.google.com/amp/s/www.thesun.co.uk/news/13855257/new-treatment-for-hair-loss-but-need-strong-stomach/amp/but when it comes to AGA they never start a human trial.
For hair cloning
Shouldn’t Breezula in women be compared to 200mg of spironolactone? I’d say that is the standard treatment in the US. (Not all of us can take that, plus it can be a sex drive killer.)
罗恩,你有任何方式做出贡献to help speed up hair therapies such as investors or mainstream media connections?
Samumed completed its phase 3 trial why the result is not out
It may take a while Praveen, patience is needed.
What do you know about the results, chief? Will they be released before summer? Thanks!
I’m not exactly sure to be honest, I keep an eye on it though.
Ron you respect Christiano? How can you respect her if she literally doesn’t work on AGA? I respect alexey T more then her, and that Follica cots guy lol. All talk from those two no action, reminds me of most politicians.
And if you’re being sarcastic about Christiano oh well I can’t delete that post lol
Actually I think this kind of info is actually what everybody needs right now (not only for stuff related to hairloss), and this website is gold, and I’ll explain why.
It is true that a cure is not around the corner, and research in this field is not being funded enough for quick results, but if you stop at this you are missing the point! You are forgetting the difference between now and 20 years ago, meaning digitalization and globalization.
I am not even talking about people losing hair, I am talking about how this is the golden age for scientific research as sharing information is so much easier than a couple of decades ago, and the digital tools we gather and develop permit to run exponentially faster simulations.
That is, if something related to hair follicles will come up in completely different centers of research as a side effect, the news will circulate in minutes and all the little discoveries from actual hair-loss cure seekers might connect like a network for one final discovery.
Moreover, the best way to make a cure (for anything) arrive faster is to make the researchers work easier.
This is why this website is amazing from a scientific perspective, it makes this process even smoother acting as an archive or a journal!
Keep it up OP and thank you
Yeah, it is possible to regrow hair—or to boost the health of the current thinner hairs—if the follicle is still intact.
I am suffering from hair loss from past 2 years. But I definitely try all the tips you have mentioned.
And so, Ron, do you happen to know anyone who may be interested in investing in hair growth companies? This is one of the biggest ways to make a difference.
Hairloss is the important step in human evolution .. we must celebrate it . ??
Thanks, Antoine Ethridge for www.tokobukuku.com
Thank you Antoine!
All of a sudden Triplehair removed the heading line for releasing TH-16 in the month of May. Now only it says release in 2021
well, I’m not sure whether to laugh or cry.
i guess it’ll be released in the next forever five years as any drug.
Giuliana pharma was supposed to release drug half month ago, u can guess what happened.
what about
1. TH16 (supposed to be relased this month)
2. Guiliani pharma (was supposed to be released Dec 20)?
1) Will update that this month.
2)//www.tokobukuku.com/way-316606-product-release-update-2020/This article has all the info about the Giuliani product, it was released and did not make a big splash.
what about
1. TH16 (supposed to be relased this month)
2. Guiliani pharma (was supposed to be released Dec 20)?
About TH 16 i visited theri website and they changes from”avout to hit the market im May 2021″ to “about to hit the market in 2021”
Hello admin
O dont get it
Imnpuretech health website about follica it says “phase 3 ready”. Does that mean that they have a clearance of the Fda to proceed to phase 3.Or does this mean that phass 3 is completed?Do you know when we will be having any news regarding Follica??? Greetings from Greece ??
I found some prolactin blocking tablets online. I wonder whether systemic intake of anti prolactin could do anything?
Is sm04554/dalosirvat result going to be released? Do you have any influence in asking them directly about it to them about what happened why delay
Hello Admin,
In last update from replicel, the have started phase 2 trail with multiple injections.
没有w they are improving their product. Will they use improved version in phase 3 now?
Great post! Anyone struggling with hair loss should definitely check out Mindwave. They sell energy healing audios that actually work, and they have one for hair loss that has really helped me alot…. My personal results have been legit life-changing, and everyone i recommend it to to says the same thing.
Keep up the great content!
Thanks for the kind words, Jamie, but your link had to be removed though in order to prevent potential spam.
First the positive:
it is really amazing how many research teams and how fast the research is now progressing in relation to clinical trials for hair loss. We seldom saw such promising approaches, consider that SM04554, for example, promised “only” 10% increase from the start, as did many others, which now failed (Fol 005).
nevertheless it seems to be 5 years away again.
Breezula is still silent, TH 16 has been postponed and the promise to publish the results of SM04550 after 6 weeks has not been kept either.
for me it will be too late by then. I hope that I am at least suitable for an HT. a (half) bald head just doesn’t suit me!
Hello
i am 50 years old woman and am suffering from thin hair since long time .. its gentic .. i did alll kinds of treatments .. am dreaming of a day to see a full healthy hair .. please i couldnt read much on your page but i will appreciate any help
Hi Rania, in short there are many treatments in development. You can follow their progress on the Ultimate Guide to Hair Regeneration article and by subscribing for email updates.
Yoda I’m having anxiety so you know I’ve been on fin now for almost 4 years it will be 4 year in mid August so I think I’m started to thin in my frontal area and crown but it’s so slow it been hard to tell but I feel like it’s then because the first I got on Finasteride I saw very good regrowth I looked back at my regrowth result pictures from 2018 and it’s not the same as of right now it’s thin. So I’m really thinking I should get in contact with my doctor and get on dutasteride but I’m scared to death what if dut doesn’t work for me at all and I just risked jumping off Finasteride. My anxiety is to much.
That was the misstake i made aswell….
Hey admin, can we get an update on stemson and moongene’s crispr? Thanks very much!
Hi flyingfish, I would love to, but there isn’t one available at the moment. Thanks for reading and stay tuned!
hi FT, i don’t understand this part of research phase why does the pig testing and mice testing take so long? After they grow the hair on mice after a couple of weeks or months what are they doing after? That time frame after they test on animals and it works… after the hair grows on animals just staring at the animals cloned hair in amazement for years? I’d like to know what these hair researchers do all day that takes a million years to get a human trial.
I don’t know exactly what goes on either woofy, and these situations vary from company to company but there are things like preparing documentation to file with the FDA. There are also toxicity studies that need to be done, or likely tumorigenicity in Stemson’s case where they need to show the safety of implanting stem cells in human tissue. All of these things do not get publicized as news on their website.
Hi woofy97,
As a molecular biologist working on stem cells and pharmacology, I can tell you that what we publish very often is either not reproduced in humans or fails to show robust responses. The reasons for this are many, but one challenge is that model organisms, such as lab mice, for the most part, lack any genetic diversity, and so when pathways and drugs are applied to the humans and the general population which has lots of genetic diversity, results are rarely as robust if at all the same as in rodents. That said, humanized mice and cells are now available, but I as I am a rapidly balding scientist in my late 40s, I am not optimistic in my lifetime that we will see a drug or application that will regrow a head of hair any better than minoxidil, derma rolling, dusteride, etc… If I do, maybe I will be able to grow hair just before the bell tolls for me.
I think we might better off trying to understand why losing our hair is so traumatic, and then trying to get over it, or at least better accept that hair loss is the cross that many men will carry. Period. If you are confident, and accepting of it, then women don’t see it. If they do care, then maybe you are better off with someone else who is not obsessed with something that, in the end, matters very little on happiness and a having a fulfilling connection with another person.
Joe
嗨乔谢谢你的评论很有趣。You never know though we may see a oral medication or injection like what Dr. Rassman is working on with human hairy nevus to grow a full head of hair that has more potential than any new potential drug because it’s unique because it’s a human extracted protean that regrows hair on mice so they have every reason to believe it will work on a human when injected into the scalp. and I’m not really into life long oral treatments, but If oral jak inhibitors worked for AA i don’t think it would impossible for another oral medication to do the same thing but for AGA. Also I think hair cloning will be available in our lifetime If it doesn’t fail.
Where can I find information about this dr ?
He’s on Reddit username is u/wrassman and look him up on google.
ah interesting. Thanks FT
This is helpful. Thanks
Hi everyone,
Does anyone have any familiarity with this new QR 678 ointment out of India. They’re making some hefty claims, but I’m getting a bit of a snake oil vibe. Any additional info or insights on this product?
Someone had recently asked in an article Nich. It’s been out for years but it’s hard to find anyone who says they tried it and it worked. I do not recommend anyone to spend time looking at it, but do as you wish.
Got it, thanks for the info!
Hey Admin, I think u might want to adjust the pyrilutamide forecast to 2022.
Found a document on reddit for Kintor stakeholders which forecasts sales for it in 2022
http://pdf.dfcfw.com/pdf/H3_AP202007241393752560_1.pdf
I appreciate you sharing but for now I’ll keep the projections at 2023 and 2024 and see what happens over the next 6 months ? If it comes in 2022, all the better.
Are there any prospects in cheaper efficient hair transplants. I’m 22 and lost most of my hair and it’s really been affecting my social/relationship life. I hope some good news comes soon cuz honestly I’m really depressed.
There are other hair transplant vetting websites out there, too be honest cheap and good hair transplants don’t really go together.
Hi yoda I feel like I’ve asked you this question before but it’s been a while and can’t remember. some people were saying if you switch from fin to dut and you got regrowth from fin but they said don’t expect any regrowth from dut just stabilization Is that true?
Hey Admin and everyone, sorry to bother once again,
due to this study (https://www.jaad.org/article/S0190-9622(21)00418-7/fulltext), oral minoxidil seems to become a more and more relevant option.
然而外用米诺地尔不帮助每个人s some people just don’t seem to respond to it. Do you know if oral minoxidil ca help those non-responders or is it simply a more conveniant alternative for people who rather take a pill than putting foam on their heads?
Many thanks in advance!
I am very happy to see this post because it really a nice post. Thanks
https://pubs.acs.org/doi/10.1021/acsnano.1c05272
Admin, do you have any speak about TH16 or sombody all? Its now avaible?
According to a reader the product is delayed. Keep an eye on recent article comment sections for news.
Triple Hair Therapy-16 is shipping and can be purchased here,https://rizn.ca/shop-rizn/.They changed the name of the Therapy-16 to Rizn.
Also, if you go to the Triple Hair site,https://triplehair.ca, you will get a popup to go to the Rizn site.
Do we think verteporfin will only be used in conjunction with hair transplant or the injections themselves could be used to revive hair follicles? As a female w diffuse thinning, I hope for the latter.
Hi Hair hopeful, veterporfin will probably just be used for hair transplant surgery. The injections themselves can’t revive miniaturized hairs you would need a wound deep enough to cause scar tissue.
I believe that verteporfin will only be effective for hair when the tissue in a wound healing process.
Interesting new information on Hope Medicine.
Article from Peking University including a picture from the macaques:
https://mp.weixin.qq.com/s/QFuAKNzmkNRhVWY7xOYq3Q
Chinese Blogger James Allen also posted about this an was able to get a reply from Xiao with not much information though, he wrote a few articles:
https://mp.weixin.qq.com/mp/appmsgalbum?__biz=MzIyMjU4Mjk3Ng==&action=getalbum&album_id=1498847593517367299&scene=173&from_msgid=2247488040&from_itemidx=1&count=3&nolastread=1#wechat_redirect
Maker the sites are not opening.can you throw some light over the information you got from these sites that you have mentioned
You can find the links on Hairlosstalk too, the pictures show macaques with insane regrowth in the first link and in the second link a Chinese Blogger writes about how big of news that is.
I’m excited to see Dr Rassman hairy nevus treatment. Looking at what a hairy nevus can already do to a human, it’s very exciting for AGA. It may actually work on a NW7 there is a good chance.
Hi Follicle Thought, I have a question about what Dr. Longaker said. He said that they would initiate a human clinical trial on verteporfin after the pig study. Then he said that he anticipates a human study of verteporfin “within 2 years” – does that mean the clinical trial will start within 2 years, or that it’ll be finished within 2 years after it starts (presumably within the next few months)? Thanks!
Tom, I believe he means to start the trial within 2 years. Thanks
The quote does sound like that, but the April 2021 New York Times article about verteporfin said that he was hoping to start human trials later in 2021. I hope nothing happened that would delay the study.
When you follow up with him about the pig studies, can you also ask him for clarification on whether he meant the human studies will start within two years, or take two years?
Thanks!
He definitely meant start human trials, and whenever I talk to him next I’ll see what his progress is.
Hello everyone, so does anyone know what the current, best treatment for hair loss is?
Hi Big dawg, the options mostly are minoxidil, finasteride (topical or oral), and hair transplants. People also combine microneedling into their regimens. There’s not really a clear consensus of what is best, but with a list so short, it’s not hard to find out what works for you with doctors guidance.
Ft do u know of anyone that is going to get a procedure done soon and using veterporfin? Or any doctors willing to use it off label?
I don’t know of any who is going to use it soon, I am in contact with a woman who says she found a plastic surgeon who has agreed to use it on her. I believe it will be for a body scar revision procedure. I will share any new I receive and it could be around 6 months for an update.
Do you know if she has had the procedure yet?
Last I heard it was set to be around Q2 2022.
Hi FT, i thought I’d share this with you and others something different then my usual posts so I found another hair wig system maker that actually in my opinion makes extremely realistic hair system it’s not the mom and pop hair system place or hair club wigs with the unrealistic hairlines this , guy I found on Instagram made a wig for rami malek who plays the villain in the new James Bond film so if you see the new James Bond film his black hair is actually a full piece wig not a partial in the film . Pretty amazing right?https://www.instagram.com/samuel_james_wigs_/This is the guy that makes the realistic wigs I don’t think he makes them for people with hairloss I think he just makes them for tv, movies and plays. There is another guy that worked with him to make rami’s James Bond wig he actually knotted the wig and his Instagram account ishttps://www.instagram.com/hand.made.wigs/and I think he makes wigs for anyone if you DM him but idk.
https://www.instagram.com/p/CTOU7YAn3qu/Check this one out FT. Looks legit. Your thoughts please?
Looks good for a wig. They’re not something I’m really interested in but could be useful to people who want/need a quick fix.
Any news
When you see Jeff Bezos with some fuzz, you’ll know that there on to something.
@Rollo yeah, cuz the richest man in the world offers himself as a lab guinea pig for a treatment of which nobody know the side effects of the long term, right
hey admin forums they say Pyrilutamide,breezula,rch-01 is not working very well and there is no hope.min/fin they say there is no alternative.
Hi nnmdm, RCH-01 was not particularly impressive in its first trial, but it has entered a new one with multiple injections. Perhaps the multiple injections will make it worthwhile. As far as pyrilutamide and breezula, we can say that Kintor is confident enough to begin a phase 3 trial for pyrilutamide whereas Cassiopea has not had the same confidence just yet. There’s always hope, even if we don’t get a game changer in the next step we are continuing to make progress.
@nnmdm fin and Minoxidil have more religious extremists on their side than Talibans lol
It seems like it is written in some holy book that they must be the only solutions lol. They are just drugs, until 1990 they barely existed, there’s no logic behind the idea that better drugs can’t be developed, maybe even starting from those two.
Hi Follicle Thought, do we have an update on TissUse?
I read the article since 2019 when they said that clinical trials are close, but we haven’t heard since.
I just posted a message in the comments of that article, Jade.
Thanks Follicle Thought, it’s unfortunate that their clinical trails would get delayed. In your opinion what are some treatments that might become available in the next 2 years?
Please do an internet search for Ultimate Guide Hair Regeneration. Thanks
Hello Folliclethought i’d be interested in trying that product for itchy scalp.
Thanks Nhan, the page should be up soon!
Hey follicle thought,
Any updates on Kintor releasing the data from their phase 2 pyrilutamide trial?
thanks!
Josh, the data is expected within the next month or so in my opinion. It will show up out of nowhere when it gets here.
@folliclethought—thanks for the quick reply. Gotta be patient! Have high hopes for this company!
Hello Folliclethought , I want to try that product for itchy scalp.
Thanks Abid, there was a slight delay in getting the signup form created by the company. It should be done on Monday, I imagine.
Hi folliclethought, is there any update on the verteporfin pig studies? It seems to take a long time. I thought the pig studies would have been published by now.
没有t yet Michael
Slight delay on the signup page from the company, perhaps should be completed by Friday 12/17.
Is Kintor waiting for Xmas to release the final data? Lol
If they are good it would be a more than appreciated gift ☺️
Dear follicle thought,
Are you ever considering starting your youtube channel again? I am sure many of us will follow you there.
Thanks for all you do,
Josh
I plan to get a few new things going in 2022, Josh. Thanks for your interest.
Hi Follicle Thought,
Are TissUse still pursuing the hair cloning treatment?
Yes, i believe they are Jade.
The result of kintors covid pill has come out and it was disappointing . Right now im starting to think that the same thing would happen to kx 826
?
@Reza just read it, let’s not despair. First, that’s a completely different drug for a completely different illness, a very recent one that is challenging even seasoned big pharma such as Pfizer and J&J. Proof is that in many countries, even with a 85/90% of vaccinated people they are reintroducing confinements and restrictions and infections are again on the rise.
Plus, I’ve just read the article and it doesn’t say very much about the drug efficacy per se but rather it complains about the lack of satisfactory clinical data, I mean, the same company said that.
I don’t really know what this mean or what happened, I leave it to the experts in the field, let’s stick with hair loss, let’s wait for the data and then we’ll discuss it ☺️
Arias—Agreed; no point in comparing two entirely different drugs. I am actually a bit annoyed by the despair and “doom-mongering” within our community. How often do we hear these memes: “they managed to go to the moon, but they can’t find a cure for hairloss?!”. Or: “they have been saying a cure is coming for the last 20 years. I guess this promising new drug will be here in 2245 guys!”.
Why won’t we simply judge the science on it’s merits? We cannot compare Kintor with cassiopea or Replicel with Amplifica; it is not fair towards the hard work that these people put in it. In fact, this type of thinking is also unfair to the goal that Folliclethought is trying to reach: provide hope and rationality towards the problem of hair loss. We have to stay level headed and rational; even more so because this industry is full of snake-oil salesmen and deceit.
Awesome comment Josh
@Josh: you see, I understand that for a person who suffers from hair loss even waiting 5 years looks like an eternity, I can perfectly understand that.
Still though, the history of hair loss medicine starts no more than 30/40 years ago, it is to say the first (rudimentary) transplant techniques, then Minoxidil, finasteride in the 90s. Ok, maybe the last 15/20 years in particular haven’t been very fruitful but science is not a straight-line process, and when you think that up to the 70s (that sound far but they’re not!) there was absolutely NOTHING, well…
As for the “they have been saying a cure is coming for the last 20 years” stuff well, this is simply not true, a quick search on Google will prove it, until 2010 there were just three or four articles, interviews with random scientists promising stuff. Way different than the many treatments concretely in the pipeline today!
It’s a complex issue, we cannot expect to wake up next morning and see people rallying in the streets like “rejoice People, baldness is over!” lol. Other illnesses have successful cures that sum up medicines and discoveries of very different periods, even centuries! We haven’t lived it and we think that they all appeared out of the blue! If they discovered a cure for baldness now, in a few decades people would say “wow, they cured baldness in just 30/40 years!”
Arias– Apologies if this is not true, but I have the feeling that you do not realize that I agreed with you. I am not sure if you understood my post. You seem to be responding to the memes that I get sick of, as if these are valid points. Again, correct me if I am wrong in that.
josh
@Josh oh no, absolutely, I got your point! I was actually replying, “ideally”, to those ones you pointed out☺️
Sorry if I didn’t express that clearly ☺️
Arias–Ah I see, my bad. Well, let’s hope that those who have these concerns can find some comfort in your comment.
I am sorry but i was already loosing hair in 2010, i started shedding around age 17. I am a woman …. I’ve been googling hairloss cures for almost 20 years. In 2005 they were already saying the cure was barely 5 years away….
Hi follicle thought,
That’s good news from Technoderma.
Do we have any idea how this drug would work and if it’s topical or oral?
Are they aiming to create a side effect free drug and are they trying to stop hair loss or regrow hair as well?
It’s a topical Jade, that’s all I am sure of for now.
At 5:58 on this video the interviewer claims that Longaker and co. have used verteporfin on pig skin. Video was posted 26 December 2021.https://www.youtube.com/watch?v=neQeFprmlhY
Surely this asserts the use on cleft pallets is imminent?
That’s great news on verteporfin.
Follicle Thought,
我们知道如果与人类临床试验计划吗ned for verteporfin?
If yes do we know when the clinical trials would start?
We know the trials are planned, but have no way to know exactly when they will start. Should be in 2022.
Thanks Follicle Thought,
Do we have any udate on TissUse or L’Oreal/Poetis?
没有thing on TissUse. Poeitis is continuing to develop an artificial skin substitute and should be releasing an update on it shortly, however, the L’Oreal/Poeitis collaboration appears to have come to an end.
By the way, if you find the content useful on this site, consider making a contribution on our Patreon pagehttps://www.patreon.com/FollicleThought
It’s unfortunate that Poeitis and L’Oreal have ended the project.
Does it mean that their program for hair cloning has come to an end?
It’s not certain that this means Loreals hair cloning program is done, it could be continuing.
I asked kintor for the results of thir phase 2 trial and they said:
Leading PI of this clinical trial is going to give a report of Phase II result on a conference middle this year. We will not release the data until then. But the Phase II primary endpoint was met.
We have started Phase III trial now, the first patient enrollment was on December 31 2021.
Thank you for your patience.
Best regards
IR team
Thanks Reza
Reza—awesome! Thanks for asking. That actually gives me some relief.
Dr. farjo is great at hype. In the middle of 2019, I had a bloody nose one day and I also happen to be looking at hairclone on Twitter and so I messaged Dr. farjo after I asked him a question about hairclone I asked him about will a bloody nose make finasteride less effective? and his response was “yes it will” I than asked my regular at-home Doctor (Dr. Alan Bauman) his nurse emailed me back saying “no it won’t. a bloody nose is not enough blood loss to effect finasteride” and I than emailed Dr. Shaver at Bernstein medical and she said the same thing that Dr. Bauman’s office told me. You won’t get anywhere from just cloning dermal papilla like Geoff hamilton said. good luck hairclone you’re not fooling me.
Spirited post against people who are taking an initiative to attempt to add density to people’s hair and see what happens.
But Woofy there is plenty of evidence from Intercytex and Aderans that demonstrates that DP cells can rejuvenate semi-minaturised and vellus hairs back into terminal hairs.
Millions of men each year begin the process of hair loss, so for them and those who are in the early stages of thinning, DP cell injections can potentially be an effective treatment.
Joseph/Admin
do you reckon HMI-115 will have great efficacy outcomes? what is your personal opinions surrounding HMI115?
im really excited for it because it will be able to restore my diffuse thining to complete thick nw1!
Thank you for the username if that’s sincere.
I have reasonable hopes for HMI-115, but to be honest I do not expect it to repeat the same type of results in humans which it did in the small monkey trial. At this point anyone outside of the company team can only speculate based on the business development of HMI-115 and the monkey trial results. The results they described were simply incredible, similar to mouse hair regrowth. I think the target for any new drug is to reach finasteride efficacy without the potential side effects and interaction on hormones. That’s an amazing starting point, really.
Really great points. As someone who has been overtly sceptic of therapies in trial, I think HMI holds a lot of excitement
Here is why I am excited
1. Bayer presents solid evidence in a non human primate – identical to the ones used for finasteride and RU studies (arguably the two most potent hair loss tools we have today)
2. HMI demonstrates progressive hair regrowth well past the 6 month mark after minimal injections.
3. Papers demonstrating the role of extra pituitary PROLACTIN in anagen regression
4. Its a monoclonal antibody – it will bind will very very very high specificity to the PRL receptor
5. Low side effect risk for males
6. Initiation of phase 2 and use of expensive lab models demonstrate the company really believes in the therapy
7. Lack of extra pituitary prolactin therapies or even therapies in research. Aka its a brand new approach to hair loss.
8. Works in both men and women – very marketable
9. Carried out by Chinese team
I was just wondering if clinical trials have been conducted with Revivv, and what the results were?
没有clinical trials as of yet, just open data collection from Dr. Rapaport. There may be some sort of trial done in the future. If/when I get info on that I will share in the Revivv thread. There’s about 100 patients using Revivv now and plenty of data forthcoming.
hi , any update from hairclone . are they going to offer dp cell injection next month ?
Hi there was actually a HairClone update a few days ago with Dr. Farjo, please review.
2022 is really an exciting year already. Kintor making smooth progress in their pyrilutamide studies and starting trials for their androgen degrader.
siRNA probably being released this year and more startups than ever. Epibiotech starting trials, Dnovo being successful with their fundraising etc.
Just very curious about Breezula’s clascoterone… It has been so silent from their end.
Josh–I guess one of us has to change their name to avoid confusion :p
Scared is a big word and I am wondering why you would call it inevitable? Perhaps you have some news that I do not know about? Thanks!
Josh, why would you say something like that? Also, it’d be best to differentiate yourself from another Josh you are replying to.
so do we have any good news yet any company saving baldness yet I’ve been away past years as i have given up on all hope so whats the news in bald world 2022 ?
Dwain ,
Your lazy approach speaks volume to your character . Admin works hard to release updates and articles frequently to keep us in the lope. Then, you just come and say “so what’s going on in the hairloss world”
At least read a bit
Cheers
@dwain you think scientific research is like putting some LEGO bricks together and puff, here it is? Sorry to disappoint you but in the past centuries many inventions and discoveries have taken decades and tons of failed attempts before getting to a good result. Today you use that result (say, the bulb) and don’t care about all the work that it took previously, while the research on baldness is something you are following in real time. Well, 20 years are literally nothing for science!
Companies, people, researchers are working to find a cure, the best we can do is supporting them…
I have been waiting for the cure over twenty years. Intercytex – Aderans Research – Dr. Christiano – Dr. Cotsarelis – Replicel & Shesheido – Tsuji & Riken – Follica – Breezula etc. There is still no green light that the cure is around corner. tired and frustrated. I am not sure any effective treatments would come out in next twenty years.
I hear ya atommin. I’m confident that several new effective treatments will be here within the next 10-15 years. Those failures in the past help shorten the timeline for the next attempts.
Administrator ,
Is there any exclusive interviews coming out soon? Have you decided if you will start a blog on your tube yet?
Thanks for all you do
Big Chungus, thanks you. There should be an interview with CosmeRNA and dNovo within a week hopefully. Just waiting on a response from one of the companies. The YT blogging is always an option but will likely be a little while until I get it going. If a small % of my audience would donate to my Patreon page, or if people subscribed to my YT back when I asked them, the YT would likely have been going for a long time now.
Hi admin
You should consider making an update announcement . You can tell us about how to donate because most people are probably even unaware .
Thanks for the feedback Big Chungus, I will do one around the time the next significant news comes. If you are on mobile or desktop use the “find” function of your browser and search for the word “Patreon” it will show you the update containing the link. On mobile browser is should be located in the menu after clicking the three dots at the top. Thanks.
Curious why you would ask me to make the announcement, but not donate yourself Big chungus? I don’t mind because it’s always your choice, just not easy to understand. If you don’t have the resources no worries.
Thank you for this great info! I’m so delighted with this blog . it is possible to regrow hair to boost the health of the current thinner hairs if the follicle is still intact.
Hi Admin and all i have come across a Redensyl german product that is available in uk and europe it is all natural if people do not want to use the topical drugs it has varied user reports and i am thinking of trying it with microneedling here is some info.
PANTHRIX hair growth activator tonic Redensyl Effectiveness: The effectiveness of Redensyl has been proven in clinical tests and studies.
Supports natural hair growth: Redensyl stimulates blood circulation, which supplies the hair follicles with important nutrients and vitamins.
Award-winning active ingredient: Redensyl® received the In-Cosmetics Silver Award from the In-Cosmetics Global Group in 2014 for innovative active ingredients
Hormones: The active ingredient has no effect on the hormonal balance
The first (measurable) results can be expected after about 6-10 weeks, then the changes begin to become visible. Shedding is possible during this growth phase.
The practical dosage form, the fine and fresh fragrance and the excellent tolerance make it pleasant to use.
PANTHRIX hair growth activator tonic Redensyl application
Apply 5–6 doses each morning and evening directly to the scalp. Gently massage the solution into the appropriate areas. Do not rinse. After taking effect, the hair can be styled as desired.
没有te on the mechanism of action
Redensyl® reactivates inactive hair follicles and thus promotes hair growth. After about 2–4 weeks of continuous use, the scalp has got used to the active ingredient complex Redensyl® and the hair follicles are strong enough to produce new hair substance outside of the skin layer. The hair is in the growth phase and is increasingly gaining length and density. The first (measurable) results can be expected after about 6–10 weeks. The changes then begin to become visible. The progress of the process can also depend on the genetic disposition and several environmental factors (lifestyle, diet, etc.). For this reason, Panthrix® should be tested for at least 3 months before the results are assessed.
PANTHRIX hair growth activator tonic Redensyl Shedding
Shedding (Engl. To shed lose) designates the short periods of strong but harmless hair loss as part of the successful topical hair growth therapy. Due to the successful treatment, the hairs with a short life expectancy fall out in the telogen growth phase and give way to the new hairs in the anagen growth phase, which are not only thicker but also live longer.
It seems like everyday there’s another redensyl product showing up to the market with the exact same info that was released in 2014 by the company (Induchem) that owns the patent on it, yet the pictures are still very scarce. Let’s see what happens with the trials of people that are now using Revivv at least the pictures look very promising and are backed by a real doctor
hey Admin,
any new updates coming soon? sadly i think all we will see are these bad OTC products, in the near future.
你说的是什么产品?CosmeRNA吗?嘿,我们把what we can get. Many people are very interested to try it.
I understand the constant interest in more news, but just for perspective this year has already surpassed many previous years I remember in terms of news, already within 4 short months. News will come when it’s available. Kintor data in June, Epibiotech potentially starting a trial in late Autumn, those are a few horizon points.
What do you mean “all we will see”? Like in the coming years or tomorrow?
Thanks for all you do ADMIN. I used to throw up bants at hairlosscure2020 but I find your blog is much more aligned with biotech and upcoming candidates . Sadly that blog turned to the side of “laser caps” and presenting studies of topical fin
Thanks for the support Prolactinogenic, but are you saying you used to post disparaging remarks about FT?
没有t at all! I just meant I used to converse with the lads over there – until I discovered FT . Now I never look back
Oh ok no problem lol, just couldn’t understand. Thanks for the support.
Hi Admin, hoping for your opinion on whether minoxidil accelerates the aging of skin through increased production of prostaglandin E2 (PGE2). This would explain the deterioration of the skin under my eyes, that I’ve noticed in the last 6 months (been on 1.5mg of oral minoxidil for just over 1 year). I really don’t want to get off minoxidil unless there is a good case for it accelerating aging.
Bree, I cannot say I have a medical opinion on this, but I have heard a reputable hair surgeon say that he believes minoxidil at least contributes to skin aging. But he didn’t mention PGE2, he mentioned that it involves the breaking down of collagen.
My comment didn’t show up for some reason. Look up studies related to scarring disorders like keloids and hypertrophic scars. They result from an overproduction of collagen, there have been studies done to use minox to intentionally break that collagen down. I think they had less than stellar results but I’m not a scientist. May be worth you guys checking some out yourselves. It made me feel comfortable enough to try minox, maybe it was confirmation bias at the time
Great blog. Hoping for the best this year. Some great news.
some shampoo or cosmetic product could be the solution?
A shampoo is not going to be the solution. Products and even drugs are pieces of the puzzle of a solution. Something like Stemson could be a solution and yet is a bit of an involved procedure (hair transplant surgery). Everything with efficacy has a place and a purpose.
Has there been any news about Kintor? I’ve read back in March that they are working on a Phase 2 trial in the US and also testing it out on women with alopecia, but haven’t heard anything since then.
你知道当他们将分享他们的结果吗?
Yes they have announced that they will be presenting their phase 2 results in June. Someone had recently shared the link with me about that, and are welcome to share it again.
Edit: found ithttps://www.marketscreener.com/quote/stock/KINTOR-PHARMACEUTICAL-LIM-111325374/news/Kintor-Pharmaceutical-Pharma-Roadshow-Presentation-39894865/
Hey Admin
I’m looking at the D Nature pipeline you just posted.
If I’m reading it right was a cosmetic released in 2020 and a new one will be released this Q3?
Was there any name/reviews for the “cosmetic 1”?
斯凯岛,据DNature告诉我什么,three pipeline candidates are all CMX, but in different concentrations. For example, cosmetic #2 and the drug will likely be higher than the first cosmetic. Yes, the first cosmetic was released as Anacell. It produces a modest hair growth effect, so it’s not my intention to overhype it. I’m sharing it as interesting information. But it does exist for sale on the internet. Note, I mentioned I’m hoping for an update about a potential improved version (cosmetic #2), though of course it will still be relative to Anacell.
Thanks Admin, was having a hard time finding anything about it online. Amazed at how you can compile all this news so quickly.
I eventually found a seller, but they don’t ship to my country.
One interesting thing though was that the application could be daily or 3 times a week.
Wonder if that means the gains are a little bit more permanent than something like minoxadil, of if that’s just a cosmetic not needing to uphold a strict dosage regimen.
Will be interesting to hear about the potentially improved version.
Anyways thanks for the response
very knowledgeable article. thanks for sharing
So admin, what do you think is the best hair regrowth technology, if I asked in today’s time, which has really shown great results and we can go for it? And what do you think will be the best one which can be launched this year in 2022? Thank you.
Admin, please keep going, not only this kind of sinergy between the news in the field is exactly what this industry needs (speeding up the process exponentially), but you are doing this with excellent professionalism.
没有click-baits, no fillers, no strong opinions, just healthy scientific journalism.
This will become the go-to website both for people interested and scientists. Keep going!
Thank you Lab rat! More good news to come!
Good to see JAK inhibitors making their way into the hair loss space; they might become a more frequent player.
Im also wondering when breezula will commence their phase 3 trial. I believe they were planning on doing it in the second half of this year..
I need to say, somewhat frustratingly, after following this site for the time when Replicel was on its final lap of marketing, I see no optimistic solution of hair loss anywhere. Cause of MPD, Mice, Research etc. are all the things I see scattered all around. Don’t we see ourselves over invested, given in a coming decade(s) as well we dont foresee any concrete solution, looking past records of consistent and continuous hypes, hopes and ultimately failures?
I don’t see really understand the point you are getting at Adhishek, what do you think could be done differently or what solution are you suggesting? I’m very interested to hear the latter.
To be fair, my above observation is not for this specific site. I became little late to quickly add the clarification. My comment was about the typical news surrounding the MPB, which have three integral components of hype, hope and failure.
For long the wish of absence of third component have not been answered. This wish continues and so does this cycle.
Thanks for clarification and I fixed your typo.
I didn’t take it personally your comments, my site can only deliver news.
但是我还是建议你,你的能量would be better directed towards a solution. I understand why you and others get frustrated by events in this industry, but that still leaves us with only one constructive option – to look towards remaining options in the future. The only other option is to give up, am I right? I believe I am.
So, is there any way we can be constructive which may actually help outcomes? Do you know investors? Do you have money to invest yourself? Can you get the topic featured in a global newspaper? These are some of the only things that can help, my friend.
follicle thought,
any updates on scube3? any chance of getting maksim plikus as an interviewee to your platform?
we have many questions about his research
yi lohn mah, most likely won’t have an interview anytime soon, but will have an update re Plikus and his company at some point over the next 3 months or so. Hang tight. They do have a compound which is prepping for a clinical trial.
Update: something nice for you yi lohn mah –https://spectrumnews1.com/oh/cleveland/health/2022/07/18/uc-irvine-scientists-disover-a-possible-cure-for-baldness#Also listed on the Updates page.
Admin!
Any update on pyrilutamide, do you have any insider information about the group buy people had made (of pyrilutamide)and its efficacy?
Why are they stalling on disclosure of the phase 2 results?
#There are some reddit threads which elaborate about the group buys people have made from a lab in China .
But apparently, those users of pyrilutamide aren’t giving any update about their efficacy.
Would glad to know your insights regarding this.
In this case of scube3, how long would the clinical trials take? thank you very much for your excellent work
I can’t say at the moment Augusto, but I will do my best to give an update before the year is out.
So frustrating that maksim plikus didn’t answer the question on when it’s likely to come to market
I will likely have an update on that within about 2 months Bree.
Admin!
Any update on pyrilutamide, do you have any insider information about the group buy people had made (of pyrilutamide)and its efficacy?
Why are they stalling on disclosure of the phase 2 results?
#There are some reddit threads which elaborate about the group buys people have made from a lab in China .
But apparently, those users of pyrilutamide aren’t giving any update about their efficacy.
Would glad to know your insights regarding this.
你好,查理,我没有具体的信息questions you’re asking. I’m awaiting to see the phase 2 results like you. I honestly don’t follow the group buy stuff like most people do, I don’t find it to be overly scientific and definitive.
Hei Rod!
Thanks for taking your time to spill some beans on group buy.
So, we can safely assume 2 things from your experiment.
The topical you are applying isnt pyri but something else.
Or
Pyri is not effective against hairloss.
Fingers crossed…
Hey Charlie ,
我是第二组的一部分,买一个of the biggest . I’m happy to be a whistleblower , despite being instructed by the mods not to do so on the discord servers. At the moment , nobody has seen any significant gains (at the 5 month mark). To be honest, I question the validity of the topical as well as of the drug itself
Cheers
Rod
Rod—. Pyrilutamide is an androgen receptor antagonist. We should be judging it based on it it’s ability to prevent hair loss, rather than it’s ability to cause significant hair growth.
Some notice about follica?
没有thing about Follica, we know it is a proprietary microneedling device plus minoxidil. Not necessarily something to hang on edge for.
@Josh: I would kill to have something that worked as well on grey hair (I suffer from premature greying but no hair loss) as minox and Finastride does for hair loss.
Raj—hey raj, yes I understand that. I guess if you don’t loose any hair you could paint it no? Good friend of mine has naturally black hair and is now painting it every now and then at the barber shop; looks great and natural in my opinion.
Josh, I don’t see that as a solution. It has many problems- if you have short hair it doesn’t work and is obvious, it’s not good for your hair and looks bad in most cases, roots come in quick, etc.
Paint is as bad a solution as Toppix or something like that in my opinion.
A clinical treatment is very long overdue. There are kids in that are 19 years old that go grey.. I don’t understand how there is no effort to fix a problem like this.
不管怎样,我希望有未来改进的解决方案for hair loss and solutions for greying so everyone can live more happy lives.
A
Find it weird that this doctor in the wired article says that the current treatments do not work very well. Finasteride + minoxidil works really really well. Yes, some have sides, but it is a very powerful hair loss treatment.
FT any new update on veterporfin coming?
粗直浓密的,我不相信这星期但也许下week. Haven’t heard about a check up this week.
Ok thanks FT
Woofy, there was actually an update posted just today. Please check the Updates page or Donor Study with Dr. Barghouthi page.
的两个治疗特定的祝福fit to those that have lost significant ground look to be verteporfin and SCUBE3. Thanks for bringing us the verteporfin updates, admin. It’s appreciated.
药品都是很好的维护,一些再保险growth, and brilliant for the 1% of hyperresponders, but for the rest a transplant may be the only viable option and this isn’t always possible if not enough grafts or the indivdiual is a diffused thinner.
你有任何新闻或更新SCUBE3吗?我听到from some individuals somewhere on this site that Dr Rassman, who works with the company developing the molecule, said on reddit that they were going to look to do trials by the end of this year or within a year (I can’t remember exactly). I understand there is a strict process any molecule needs to undergo when being tested for a new purpose but it looks like this one has real potential. The makers of the molecule would definitely be in a great financial situation if it ever goes to market too, I’m sure.
Thanks again, FT.
Magic City, thanks for the appreciation.
Scube3 is actually a new molecule that Amplifica may develop over the next 2 yrs. They have multiple initial compounds which are not Scube3. One of their original compounds should begin trials over the next half year or so (the one mentioned by Dr. R). I’m sure they will announce it when the trial does begin, but that’s all I know for now.
Thanks very much, good info. Didn’t know about the other initial compounds, very interesting
Thank you for the update!
Great content and knowledgeable information shared. Thanks and keep it up
I NEED SOME TIPS ON MY HAIR. It was a good read but ive been struggling for a few years and my hairline is fucked. Please help me restore my hair line.
Griffin Shaw.
Griffin—what are you doing to prevent hair loss or stimulate hair growth at the moment?
Yes and have you researched hair transplants and where to find the best/ethical surgeons?
Thanks for your blog.I NEED SOME TIPS ON MY HAIR. It was a good read but ive been struggling for a few years and my hairline is fucked. Please help me restore my hair line.
Hey Mark—there is quite some helpful information on this website if you scroll up and go to the tab “advice”. There you will find some links to other articles regarding treatments or hairtransplants. Also on youtube you’l find some decent channels like Mattdominance, Mr.rolandas, hairlisciously etc.
By the way @folliclethought, I noticed you have not updated your “Ultimate guide to hair regeneration” page in a while. I’ve always found that a very helpful page to look at the current state of affairs regarding ongoing trials. Have you decided to not update that any further? Thanks in advance!
Josh, I haven’t updated in a while mostly due to time allotment. I will try to update it soon for you.
Follicle thought—understandable, it doesn’t have high priority. Thanks for everything.
Do you have a list of current hair growth treatments and their progress. I’m curious to see which ones are in Phase 2 or Phase 3 or their trials and will be on the market soon.
I was also wondering if you know if hair follicles are truly “dead” once they stop producing hairs. I’ve been finding conflicting information online, some saying that once a hair follicles stops creating hairs, the effect is permanent, while others say it is possible to revive them.
Jean-Luc please do a google search for “hair regeneration 2022” and it should bring you to my guide article with more pipeline information and timelines.
@Folliclethought, In that Wired article Dr. Maksim Plikus said and I quote, ”the therapy would probably need to be repeated two or three times a year to ensure continued hair growth. “Pharma would love the model,”
What do you think he meant by ”a year”? As in you’d have to have a couple of injections a year every year? By the way, have you thought interviewing him for your blog?
I think it’s multiple injections every year, since he compares it with botox with also needs regular repeated in injections…
Cygnus, So basically for the rest of your life?
是的这就是我认为从引用的the article at least. Which I personally found a tiny bit of a blow because he also compares the projected costs of the treatment, to the cost of botox injections so it’s ruled out of me personally because in the case of my country it would be more expensive then a high end hair system per year… But hey I try to stay open & optimistic maybe one day the basic SCUBE treatment being developed now can be improved even further therefore reducing the injection frequency & costs
While it’s great that SCUBE3 has been revealed now, it will be years before it is available for us (if it even reaches that stage following trials). Apparently, the company Maksim Pilkus is working with to develop SCUBE3 is developing other compounds first and before SCUBE3 so this will likely put an even further delay on this treatment coming to market.
I quote Follicle Thought comment above from 15 September 2022:
‘Scube3 is actually a new molecule that Amplifica may develop over the next 2 yrs. They have multiple initial compounds which are not Scube3. One of their original compounds should begin trials over the next half year or so (the one mentioned by Dr. R). I’m sure they will announce it when the trial does begin, but that’s all I know for now.’
In my opinion, we are talking at least 5 years here.
I think it’s best to keep focused on more immediate and upcoming treatments like verteporfin and pyrilutamide.
Magic City,
Yes, SCUBE3 is years away. Although the prospect of having several expensive yearly injections doesn’t sound particularly appetizing. Unless regrowing your hair using Scube (or some other wonder drug) and then trying then to maintain it (by using pyrul or some other drug that doesn’t have side effects) with cheaper meds, that I could go along with. Sort of like having a hair transplant and then maintaining the new hair with Fin, but in a much more minimally invasive way.
By the way, it’s kinda shocking that Min/Fin are the only medications that we’ve had for the last 25 years to combat this condition. I hope we’ll have much better treatments (pyrilutamide could be one of them) than Min/Fin/Dut by the end of this decade.
Follicle thought, I was browsing through World Congress for Hair Research 2022 Congress Program and saw something that might of interest. The name of the presentation is ”Therapeutic effects of the application of a novel growth factor cocktail solution (Moliniq™) via microneedling on the scalp of patients with androgenetic alopecia: A split study”.
http://article.scholarena.com/Therapeutic-Effects-of-the-Application.pdf
Thanks Alexis, will look into it.
Update: The trichograms were limited in number and the product not seem overly promising to me, but if they send me more information I will take a look.
The reason I mentioned it is because it had similar results (20.2 hairs per cm²) to KX-826, which showed total increase of 22.73 hairs per cm². Sadly, that’s the only information I could find.
A few years back you had the Tesla Brush as a recommended product on your site, but I noticed that you removed it. I looked through the updates page to see if you’d written anything else about it or microcurrent in general, but haven’t seen anything.
Would you mind sharing any additional insight you have into that treatment modality and the tesla brush in particular?
I sincerely believe that all of us should have hope in Junji Fukuda’s work. Probably just like me, we have all had hopes in the solution of baldness with companies that have failed for so many years, but with Fukuda what I see is a defined timeline and little by little it is fulfilling its objectives. According to his predictions, he planned to carry out a human trial next year and there are still 14 months to go (at least for more updates on his work) and so far he has managed to determine the mechanism of organogenesis of a follicle, cultivate it in an environment outside the human body, to develop a method to optimize capillary multiplication for patients who need a large amount of hair and how it will be possible for the hair to grow through the skin and with the correct characteristics. We still have time to be skeptical and to affirm that he has failed but according to his work and his timeline I trust Fukuda (:
是的,Teslabrush停止培养什么样的主人g it independently and I could no longer offer it. I don’t have much to share other than that I believe microcurrent is a reasonable modality which can produce some benefit, especially when engineered/crafted with good expertise for hair growth. I hope that other companies will improve upon it and take it to market sometime.
Encouraged to see Eirion moving forward. A grey hair pharmaceutical solution in the next five years would be a godsend.
Glad to see the CEO Jon Edelson specifically mention the grey hair (and hair loss) treatment in the press release. That tells me they are hopefully on track with it.